Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder
- PMID: 19375091
- PMCID: PMC2730562
- DOI: 10.1016/j.juro.2009.01.117
Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder
Abstract
Purpose: We determined the effectiveness of cystoscopic administration of botulinum-A toxin compared to placebo for the treatment of urinary incontinence in subjects with idiopathic overactive bladder.
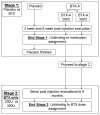
Materials and methods: Subjects were recruited from the Division of Urogynecology at the University of Rochester. Inclusion criteria were overactive bladder refractory to anticholinergic medications, multiple daily incontinence episodes and a 24-hour pad weight of 100 gm or greater. Subjects with low leak point pressures, increased post-void residual volume or neurological etiologies were excluded from study. Subjects were randomized to placebo or to 1 of 2 doses of botulinum-A toxin. The detrusor was injected at 8 to 10 sites above the trigone. Evaluations were performed at baseline, and at 3 and 6 weeks after injection, and included bladder diaries, pad weights, quality of life questionnaires and urodynamic studies.
Results: A total of 22 subjects participated in stage 1 of this 2-stage study. We report on the outcomes of stage 1 of this study. Because stage 2 is still ongoing and investigators remain blind to the doses of botulinum-A toxin, the 2 botulinum-A toxin groups were combined for this report. There were no differences in mean baseline measurements between the 2 groups. Statistically significant improvements in daily incontinence episodes, pads changed per day and quality of life questionnaires were seen in the botulinum-A toxin group with no changes in the placebo group. No change in nocturia, daily voiding frequency, peak flow or detrusor pressure was seen in either group. Of 15 subjects 4 (26%) receiving botulinum-A toxin had a post-void residual volume of 200 cc or greater and 1 subject required intermittent catheterization. Four subjects experienced a urinary tract infection, 2 (13%) in the botulinum-A toxin group and 2 (28%) in the placebo group (not significant).
Conclusions: Botulinum-A toxin can significantly reduce urge urinary incontinence due to overactive bladder at 6 weeks. However, there is a risk of urinary retention requiring self-catheterization.
Figures
References
-
- Weber A, Walters M. Urogynecology and Pelvic Reconstructive Surgery. Mosby; St. Louis, Missouri: 1999. Epidemiology and social impact of urinary and fecal incontinence; pp. 25–33.
-
- Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002;168:1897. - PubMed
-
- Aoki KR. Pharmacology and immunology of botulinum toxin serotypes. J Neurol. 2001;248:3. - PubMed
-
- Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle Nerve. 1997;6(suppl):S146. - PubMed
-
- Jankovic J, Brin M. Botulinum toxin: historical perspective and potential new indications. Muscle Nerve. 1997;6(suppl):S129. - PubMed