Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord
- PMID: 19375225
- PMCID: PMC2693265
- DOI: 10.1016/j.pain.2009.03.005
Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord
Abstract
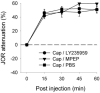
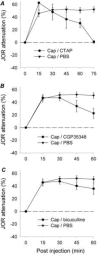
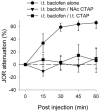
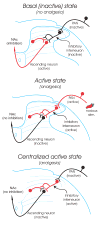
The duration of noxious stimulus-induced antinociception (NSIA) has been shown to outlast the pain stimulus that elicited it, however, the mechanism that determines the duration of analgesia is unknown. We evaluated the role of spinal excitatory and inhibitory receptors (NMDA, mGluR(5), mu-opioid, GABA(A), and GABA(B)), previously implicated in NSIA initiation, in its maintenance. As in our previous studies, the supraspinal trigeminal jaw-opening reflex (JOR) in the rat was used for nociceptive testing because of its remoteness from the region of drug application, the lumbar spinal cord. NSIA was reversed by antagonists for two inhibitory receptors (GABA(B) and mu-opioid) but not by antagonists for either of the two excitatory receptors (NMDA and mGluR(5)), indicating that NSIA is maintained by ongoing activity at inhibitory synapses in the spinal cord. Furthermore, spinal administration of the GABA(B) agonist baclofen mimicked NSIA in that it could be blocked by prior injection of the mu-opioid receptor antagonist H-D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH(2) (CTAP) in nucleus accumbens. CTAP also blocked baclofen antinociception when administered in the spinal cord. We conclude that analgesia induced by noxious stimulation is maintained by activity in spinal inhibitory receptors.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures
Similar articles
-
Contribution of spinal inhibitory receptors in heterosegmental antinociception induced by noxious stimulation.Eur J Neurosci. 2003 Dec;18(11):2999-3006. doi: 10.1111/j.1460-9568.2003.03031.x. Eur J Neurosci. 2003. PMID: 14656295
-
Inhibition of pain behavior by GABA(B) receptors in the thalamic ventrobasal complex: effect on normal rats subjected to the formalin test of nociception.Brain Res. 2006 Oct 18;1115(1):37-47. doi: 10.1016/j.brainres.2006.07.089. Epub 2006 Aug 30. Brain Res. 2006. PMID: 16938274
-
Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons.J Neurophysiol. 1998 Feb;79(2):677-87. doi: 10.1152/jn.1998.79.2.677. J Neurophysiol. 1998. PMID: 9463431
-
Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa: inhibition by mu-opioid and GABAB agonists.J Physiol. 1998 Mar 1;507 ( Pt 2)(Pt 2):473-83. doi: 10.1111/j.1469-7793.1998.473bt.x. J Physiol. 1998. PMID: 9518706 Free PMC article.
-
Spinal inhibitory neurotransmission in neuropathic pain.Curr Pain Headache Rep. 2009 Jun;13(3):208-14. doi: 10.1007/s11916-009-0035-8. Curr Pain Headache Rep. 2009. PMID: 19457281 Free PMC article. Review.
Cited by
-
Endogenous analgesia, dependence, and latent pain sensitization.Curr Top Behav Neurosci. 2014;20:283-325. doi: 10.1007/7854_2014_351. Curr Top Behav Neurosci. 2014. PMID: 25227929 Free PMC article.
-
Systemic and intrathecal baclofen produce bladder antinociception in rats.BMC Urol. 2021 Oct 4;21(1):139. doi: 10.1186/s12894-021-00899-0. BMC Urol. 2021. PMID: 34607587 Free PMC article.
-
Therapeutic Efficacy of Medicinal Plants with Allopathic Medicine in Musculoskeletal Diseases.Int J Plant Anim Environ Sci. 2024;14(4):104-129. doi: 10.26502/ijpaes.4490170. Epub 2024 Dec 23. Int J Plant Anim Environ Sci. 2024. PMID: 39866300 Free PMC article.
-
Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence.Science. 2013 Sep 20;341(6152):1394-9. doi: 10.1126/science.1239403. Science. 2013. PMID: 24052307 Free PMC article.
-
Rat model of attention-deficit hyperactivity disorder exhibits delayed recovery from acute incisional pain due to impaired descending noradrenergic inhibition.Sci Rep. 2023 Apr 4;13(1):5526. doi: 10.1038/s41598-023-32512-9. Sci Rep. 2023. PMID: 37016045 Free PMC article.
References
-
- Ahn DK, Kim YS, Park JS. Central NO is involved in the antinociceptive action of intracisternal antidepressants in freely moving rats. Neurosci Lett. 1998;243:105–8. - PubMed
-
- Banks D, Kuriakose M, Matthews B. Modulation by peripheral conditioning stimuli of the responses of trigeminal brain stem neurones and of the jaw opening reflex to tooth pulp stimulation in chronically prepared, anaesthetized cats. Exp Physiol. 1992;77:343–9. - PubMed
-
- Belforte JE, Barceló AC, Pazo JH. Striatal modulation of the jaw opening reflex. Brain Res. 2001;891:138–47. - PubMed
-
- Bouhassira D, Bing Z, Le Bars D. Studies of the brain structures involved in diffuse noxious inhibitory controls: the mesencephalon. J Neurophysiol. 1990;64:1712–23. - PubMed
-
- Bouhassira D, Bing Z, Le Bars D. Effects of lesions of locus coeruleus/subcoeruleus on diffuse noxious inhibitory controls in the rat. Brain Res. 1992;571:140–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

