Applications of high-throughput genomics to antiviral research: evasion of antiviral responses and activation of inflammation during fulminant RNA virus infection
- PMID: 19375457
- PMCID: PMC3457704
- DOI: 10.1016/j.antiviral.2009.04.004
Applications of high-throughput genomics to antiviral research: evasion of antiviral responses and activation of inflammation during fulminant RNA virus infection
Abstract
Host responses can contribute to the severity of viral infection, through the failure of innate antiviral mechanisms to recognize and restrict the pathogen, the development of intense systemic inflammation leading to circulatory failure or through tissue injury resulting from overly exuberant cell-mediated immune responses. High-throughput genomics methods are now being used to identify the biochemical pathways underlying ineffective or damaging host responses in a number of acute and chronic viral infections. This article reviews recent gene expression studies of 1918 H1N1 influenza and Ebola hemorrhagic fever in cell culture and animal models, focusing on how genomics experiments can be used to increase our understanding of the mechanisms that permit those viruses to cause rapidly overwhelming infection. Particular attention is paid to how evasion of type I IFN responses in infected cells might contribute to over-activation of inflammatory responses. Reviewing recent research and describing how future studies might be tailored to understand the relationship between the infected cell and its environment, this article discusses how the rapidly growing field of high-throughput genomics can contribute to a more complete understanding of severe, acute viral infections and identify novel targets for therapeutic intervention.
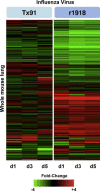
Figures
Similar articles
-
Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses.J Virol. 2015 Jun;89(12):6442-52. doi: 10.1128/JVI.00319-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855745 Free PMC article.
-
The 1918 Influenza Virus PB2 Protein Enhances Virulence through the Disruption of Inflammatory and Wnt-Mediated Signaling in Mice.J Virol. 2015 Dec 9;90(5):2240-53. doi: 10.1128/JVI.02974-15. J Virol. 2015. PMID: 26656717 Free PMC article.
-
NS1 Protein Mutation I64T Affects Interferon Responses and Virulence of Circulating H3N2 Human Influenza A Viruses.J Virol. 2016 Oct 14;90(21):9693-9711. doi: 10.1128/JVI.01039-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535054 Free PMC article.
-
Mechanisms of innate immune evasion in re-emerging RNA viruses.Curr Opin Virol. 2015 Jun;12:26-37. doi: 10.1016/j.coviro.2015.02.005. Epub 2015 Mar 9. Curr Opin Virol. 2015. PMID: 25765605 Free PMC article. Review.
-
Innate immune evasion by filoviruses.Virology. 2015 May;479-480:122-30. doi: 10.1016/j.virol.2015.03.030. Epub 2015 Apr 3. Virology. 2015. PMID: 25843618 Free PMC article. Review.
Cited by
-
Gene expression signature-based screening identifies new broadly effective influenza a antivirals.PLoS One. 2010 Oct 4;5(10):e13169. doi: 10.1371/journal.pone.0013169. PLoS One. 2010. PMID: 20957181 Free PMC article.
-
Tackling Ebola: new insights into prophylactic and therapeutic intervention strategies.Genome Med. 2011 Jan 27;3(1):5. doi: 10.1186/gm219. Genome Med. 2011. PMID: 21349211 Free PMC article.
-
Treatment with the reactive oxygen species scavenger EUK-207 reduces lung damage and increases survival during 1918 influenza virus infection in mice.Free Radic Biol Med. 2014 Feb;67:235-47. doi: 10.1016/j.freeradbiomed.2013.10.014. Epub 2013 Oct 17. Free Radic Biol Med. 2014. PMID: 24140866 Free PMC article.
-
Case report: incidental findings of COVID-19 infection on positron emission tomography/computed tomography for staging of a giant gastric gastrointestinal stromal tumor.Pan Afr Med J. 2020 May 11;35(Suppl 2):28. doi: 10.11604/pamj.supp.2020.35.2.23167. eCollection 2020. Pan Afr Med J. 2020. PMID: 33623553 Free PMC article.
-
Molecular imaging of influenza and other emerging respiratory viral infections.J Infect Dis. 2011 May 15;203(10):1348-59. doi: 10.1093/infdis/jir038. Epub 2011 Mar 21. J Infect Dis. 2011. PMID: 21422476 Free PMC article. Review.
References
-
- Anon Influenza A virus subtype H5N1 infection in humans. Commun. Dis. Rep. Wkly. 1997;7:441. - PubMed
-
- Anon Isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, May–December 1997. Morb. Mortal. Wkly. Rep. 1997;46:1204–1207. - PubMed
-
- Abdel-Ghafar A.N., Chotpitayasunondh T., Gao Z., Hayden F.G., Nguyen D.H., de Jong M.D., Naghdaliyev A., Peiris J.S., Shindo N., Soeroso S., Uyeki T.M. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 2008;358:261–273. - PubMed
-
- Ank N., Iversen M.B., Bartholdy C., Staeheli P., Hartmann R., Jensen U.B., Dagnaes-Hansen F., Thomsen A.R., Chen Z., Haugen H., Klucher K., Paludan S.R. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 2008;180:2474–2485. - PubMed
-
- Ank N., West H., Paludan S.R. IFN-lambda: novel antiviral cytokines. J. Interferon Cytokine Res. 2006;26:373–379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

