Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes
- PMID: 19375465
- PMCID: PMC2768481
- DOI: 10.1016/j.neuint.2009.04.002
Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes
Abstract
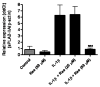
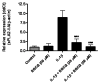
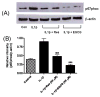
Recent studies have suggested the involvement of secretory phospholipase A2-IIA (sPLA2-IIA) in neuroinflammatory diseases. Although sPLA2-IIA is transcriptionally induced through the NF-kappaB pathway by pro-inflammatory cytokines, whether this induction pathway is affected by other intracellular signaling pathways has not been investigated in detail. In this study, we demonstrated the induction of sPLA2-IIA mRNA and protein expression in astrocytes by cytokines and detected the protein in the culture medium after stimulation. We further investigated the effects of oxidative pathways and botanical antioxidants on the induction pathway and observed that IL-1beta-induced sPLA2-IIA mRNA expression in astrocytes is dependent on ERK1/2 and PI-3 kinase, but not p38 MAPK. In addition to apocynin, a known NADPH oxidase inhibitor, botanical antioxidants, such as resveratrol and epigallocatechin gallate, also inhibited IL-1beta-induced sPLA2-IIA mRNA expression. These compounds also suppressed IL-1beta-induced ERK1/2 activation and translocation of the NADPH oxidase subunit p67 phox from cytosol to membrane fraction. Taken together, these results support the involvement of reactive oxygen species from NADPH oxidase in cytokine induction of sPLA2-IIA in astrocytes and promote the use of botanical antioxidants as protective agents for inhibition of inflammatory responses in these cells.
Figures





Similar articles
-
AMPK Signaling Involvement for the Repression of the IL-1β-Induced Group IIA Secretory Phospholipase A2 Expression in VSMCs.PLoS One. 2015 Jul 10;10(7):e0132498. doi: 10.1371/journal.pone.0132498. eCollection 2015. PLoS One. 2015. PMID: 26162096 Free PMC article.
-
Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA₂-IIA expression in astrocytes and microglia.J Neuroinflammation. 2011 Sep 24;8:121. doi: 10.1186/1742-2094-8-121. J Neuroinflammation. 2011. PMID: 21943492 Free PMC article.
-
Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain.J Neurochem. 2004 Aug;90(3):637-45. doi: 10.1111/j.1471-4159.2004.02540.x. J Neurochem. 2004. PMID: 15255941
-
Modulation of cytokine-induced expression of secretory phospholipase A2-type IIA by protein kinase C in rat renal mesangial cells.Biochem Pharmacol. 1999 Dec 1;58(11):1751-8. doi: 10.1016/s0006-2952(99)00279-8. Biochem Pharmacol. 1999. PMID: 10571249
-
Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma.J Neurosci Res. 2004 Aug 15;77(4):540-51. doi: 10.1002/jnr.20180. J Neurosci Res. 2004. PMID: 15264224
Cited by
-
Moderate blood alcohol and brain neurovulnerability: Selective depletion of calcium-independent phospholipase A2, omega-3 docosahexaenoic acid, and its synaptamide derivative as a potential harbinger of deficits in anti-inflammatory reserve.Alcohol Clin Exp Res. 2021 Dec;45(12):2506-2517. doi: 10.1111/acer.14734. Epub 2021 Nov 16. Alcohol Clin Exp Res. 2021. PMID: 34719812 Free PMC article.
-
Secreted phospholipase A2 involvement in neurodegeneration: differential testing of prosurvival and anti-inflammatory effects of enzyme inhibition.PLoS One. 2012;7(6):e39257. doi: 10.1371/journal.pone.0039257. Epub 2012 Jun 15. PLoS One. 2012. PMID: 22720084 Free PMC article.
-
Neuroprotective roles of the P2Y(2) receptor.Purinergic Signal. 2012 Sep;8(3):559-78. doi: 10.1007/s11302-012-9307-6. Epub 2012 Apr 14. Purinergic Signal. 2012. PMID: 22528682 Free PMC article. Review.
-
Regulatory effects of the JAK3/STAT1 pathway on the release of secreted phospholipase A₂-IIA in microvascular endothelial cells of the injured brain.J Neuroinflammation. 2012 Jul 12;9:170. doi: 10.1186/1742-2094-9-170. J Neuroinflammation. 2012. PMID: 22788969 Free PMC article.
-
Molecular Mechanism of Resveratrol's Lipid Membrane Protection.Sci Rep. 2018 Jan 25;8(1):1587. doi: 10.1038/s41598-017-18943-1. Sci Rep. 2018. PMID: 29371621 Free PMC article.
References
-
- Andersen JM, Myhre O, Fonnum F. Discussion of the role of the extracellular signal-regulated kinase-phospholipase A2 pathway in production of reactive oxygen species in Alzheimer’s disease. Neurochemical Research. 2003;28:319–326. - PubMed
-
- Andreani M, Olivier JL, Berenbaum F, Raymondjean M, Bereziat G. Transcriptional regulation of inflammatory secreted phospholipases A(2) Biochimica et Biophysica Acta. 2000;1488:149–158. - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annual Review of Immunology. 1994;12:141–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

