Progression of human bone marrow stromal cells into both osteogenic and adipogenic lineages is differentially regulated by structural conformation of collagen I matrix via distinct signaling pathways
- PMID: 19375503
- PMCID: PMC6817339
- DOI: 10.1016/j.matbio.2009.04.003
Progression of human bone marrow stromal cells into both osteogenic and adipogenic lineages is differentially regulated by structural conformation of collagen I matrix via distinct signaling pathways
Abstract
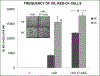
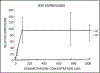
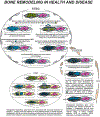
Adult human bone marrow stromal cells (BMSCs) containing or consisting of mesenchymal stem cells (MSCs) are an important source in tissue homeostasis and repair. Although many processes involved in their differentiation into diverse lineages have been deciphered, substantial inroads remain to be gained to synthesize a complete regulatory picture. The present study suggests that structural conformation of extracellular collagen I, the major organic matrix component in musculoskeletal tissues, plays, along with differentiation stimuli, a decisive role in the selection of differentiation lineage. It introduces a novel concept which proposes that structural transition of collagen I matrix regulates cell differentiation through distinct signaling pathways specific for the structural state of the matrix. Thus, on native collagen I matrix inefficient adipogenesis is p38-independent, whereas on its denatured counterpart, an efficient adipogenesis is primarily regulated by p38 kinase. Inversely, osteogenic differentiation occurs efficiently on native, but not on denatured collagen I matrix, with a low commencement threshold on the former and a substantially higher one on the latter. Osteogenesis on collagen I matrices in both structural conformations is fully dependent on ERK. However, whereas on native collagen I matrix osteogenic differentiation is Hsp90-dependent, on denatured collagen I matrix it is Hsp90-independent. The matrix conformation-mediated regulation appears to be one of the mechanisms determining differentiation lineage of BMSCs. It allows a novel interpretation of the bone remodeling cycle, explains the marked physiological aging-related adipogenic shift in musculoskeletal tissues, and can be a principal contributor to adipogenic shift seen in a number of clinical disorders.
Figures






Similar articles
-
Human bone marrow-derived stromal cells show highly efficient stress-resistant adipogenesis on denatured collagen IV matrix but not on its native counterpart: implications for obesity.Matrix Biol. 2010 Jan;29(1):9-14. doi: 10.1016/j.matbio.2009.09.002. Epub 2009 Sep 15. Matrix Biol. 2010. PMID: 19761844 Free PMC article.
-
Collagen I matrix contributes to determination of adult human stem cell lineage via differential, structural conformation-specific elicitation of cellular stress response.Matrix Biol. 2009 Jun;28(5):251-62. doi: 10.1016/j.matbio.2009.04.002. Epub 2009 Apr 16. Matrix Biol. 2009. PMID: 19375506 Free PMC article.
-
Adult human bone marrow stromal cells regulate expression of their MMPs and TIMPs in differentiation type-specific manner.Matrix Biol. 2010 Jan;29(1):3-8. doi: 10.1016/j.matbio.2009.09.003. Epub 2009 Sep 17. Matrix Biol. 2010. PMID: 19765656 Free PMC article.
-
[Regulation of the differentiation of bone marrow mesenchymal stem cells into osteoblastic and adipogenic lineage].Chir Narzadow Ruchu Ortop Pol. 2011 Mar-Apr;76(2):96-8. Chir Narzadow Ruchu Ortop Pol. 2011. PMID: 21853910 Review. Polish.
-
How is mechanobiology involved in mesenchymal stem cell differentiation toward the osteoblastic or adipogenic fate?J Cell Physiol. 2019 Aug;234(8):12133-12141. doi: 10.1002/jcp.28099. Epub 2019 Jan 11. J Cell Physiol. 2019. PMID: 30633367 Review.
Cited by
-
Sika Deer Antler Collagen Type I-Accelerated Osteogenesis in Bone Marrow Mesenchymal Stem Cells via the Smad Pathway.Evid Based Complement Alternat Med. 2016;2016:2109204. doi: 10.1155/2016/2109204. Epub 2016 Jan 4. Evid Based Complement Alternat Med. 2016. PMID: 27066099 Free PMC article.
-
Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering.Tissue Eng Part C Methods. 2012 Nov;18(11):866-76. doi: 10.1089/ten.TEC.2012.0009. Epub 2012 Jul 2. Tissue Eng Part C Methods. 2012. PMID: 22559904 Free PMC article.
-
Human bone marrow-derived stromal cells show highly efficient stress-resistant adipogenesis on denatured collagen IV matrix but not on its native counterpart: implications for obesity.Matrix Biol. 2010 Jan;29(1):9-14. doi: 10.1016/j.matbio.2009.09.002. Epub 2009 Sep 15. Matrix Biol. 2010. PMID: 19761844 Free PMC article.
-
Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation.Stem Cells Dev. 2012 Sep 1;21(13):2442-56. doi: 10.1089/scd.2011.0615. Epub 2012 May 31. Stem Cells Dev. 2012. PMID: 22455378 Free PMC article.
-
Matrix remodeling as stem cell recruitment event: a novel in vitro model for homing of human bone marrow stromal cells to the site of injury shows crucial role of extracellular collagen matrix.Matrix Biol. 2010 Oct;29(8):657-63. doi: 10.1016/j.matbio.2010.08.008. Epub 2010 Sep 7. Matrix Biol. 2010. PMID: 20828613 Free PMC article.
References
-
- Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W and Baccarini M (2000). Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem 275, 22300–4. - PubMed
-
- Altman GH, Horan RL, Martin I, and Farhadi J (2002). Cell differentiation by mechanical stress. FASEB J 16, 270–2. - PubMed
-
- Baron R (1989) Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 224(2):317–24. Review. - PubMed
-
- Birkedal-Hansen H (1993). Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol 64, 474–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

