Mesenchymal stem cells suppress B-cell terminal differentiation
- PMID: 19375651
- PMCID: PMC2747661
- DOI: 10.1016/j.exphem.2009.01.005
Mesenchymal stem cells suppress B-cell terminal differentiation
Abstract
Objective: Mesenchymal stem cells (MSCs) have been shown to possess immunomodulatory properties on a diverse array of immune cell lineages. However, their effect on B lymphocytes remains unclear. We investigated the effect of MSCs on B-cell modulation with a special emphasis on gene regulation mediated by MSC humoral factors.
Materials and methods: MSCs were isolated from C57BL/6 bone marrow and expanded in culture. Splenic B cells were purified using anti-CD43 antibody and immunomagnetic beads. B cells and MSCs were cocultured in separate compartments in a transwell system. For B-cell stimulation, lipopolysaccharide was used in vitro and T-dependent and T-independent antigens were used in vivo.
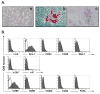
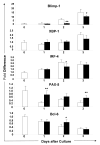
Results: In MSC cocultures, lipopolysaccharide-stimulated B-cell proliferation was suppressed, CD138(+) cell percentage decreased, and the number of apoptotic CD138(+) cells decreased. In the B/MSC coculture, the IgM(+) cell percentage was higher and the IgM amount released in the medium was lower than in the control. The B-lymphocyte-induced maturation protein-1 messenger RNA expression in the coculture was suppressed throughout the 3-day culture period. Conditioned media derived from MSC cultures prevented terminal differentiation of B cells in vitro and significantly suppressed the antigen-specific immunoglobulin M and immunoglobulin G1 secretion in mice immunized with T-cell-independent as well as T-cell-dependent antigens in vivo.
Conclusion: Results indicate that humoral factor(s) released by MSCs exert a suppressive effect on the B-cell terminal differentiation. Suppression may be mediated through inhibition of B-lymphocyte-induced maturation protein-1 expression, but the nature of the factor(s) is yet to be determined.
Figures





References
-
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. - PubMed
-
- Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene ther. 2000;7:451–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

