Myocyte remodeling in response to hypertrophic stimuli requires nucleocytoplasmic shuttling of muscle LIM protein
- PMID: 19376126
- PMCID: PMC2739242
- DOI: 10.1016/j.yjmcc.2009.04.006
Myocyte remodeling in response to hypertrophic stimuli requires nucleocytoplasmic shuttling of muscle LIM protein
Abstract
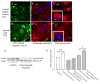
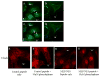
CSRP3 or muscle LIM protein (MLP) is a nucleocytoplasmic shuttling protein and a mechanosensor in cardiac myocytes. MLP regulation and function was studied in cultured neonatal rat myocytes treated with pharmacological or mechanical stimuli. Either verapamil or BDM decreased nuclear MLP while phenylephrine and cyclic strain increased it. These results suggest that myocyte contractility regulates MLP subcellular localization. When RNA polymerase II was inhibited with alpha-amanitin, nuclear MLP was reduced by 30%. However, when both RNA polymerase I and II were inhibited with actinomycin D, there was a 90% decrease in nuclear MLP suggesting that its nuclear translocation is regulated by both nuclear and nucleolar transcriptional activity. Using cell permeable synthetic peptides containing the putative nuclear localization signal (NLS) of MLP, nuclear import of the protein in cultured rat neonatal myocytes was inhibited. The NLS of MLP also localizes to the nucleolus. Inhibition of nuclear translocation prevented the increased protein accumulation in response to phenylephrine. Furthermore, cyclic strain of myocytes after prior NLS treatment to remove nuclear MLP resulted in disarrayed sarcomeres. Increased protein synthesis and brain natriuretic peptide expression were also prevented suggesting that MLP is required for remodeling of the myofilaments and gene expression. These findings suggest that nucleocytoplasmic shuttling MLP plays an important role in the regulation of the myocyte remodeling and hypertrophy and is required for adaptation to hypertrophic stimuli.
Figures






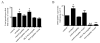
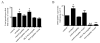
References
-
- Benmerah A, Scott M, Poupon V, Marullo S. Nuclear functions for plasma membrane-associated proteins? Traffic. 2003;4(8):503–11. - PubMed
-
- Wixler V, Geerts D, Laplantine E, Westhoff D, Smyth N, Aumailley M, et al. The LIM-only protein DRAL/FHL2 binds to the cytoplasmic domain of several alpha and beta integrin chains and is recruited to adhesion complexes. Journal of Biological Chemistry. 2000;275(43):33669–78. - PubMed
-
- Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, et al. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. American Journal of Physiology - Heart & Circulatory Physiology. 2007;292(1):H259–69. - PubMed
-
- Gehmlich K, Geier C, Milting H, Furst D, Ehler E. Back to square one: what do we know about the functions of Muscle LIM Protein in the heart? J Muscle Res Cell Motil. 2008 Dec 30; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

