Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases
- PMID: 19376264
- PMCID: PMC3652637
- DOI: 10.1016/j.preteyeres.2009.04.003
Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases
Abstract
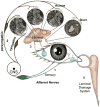
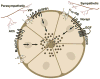
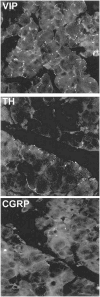
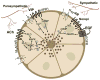
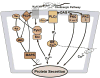
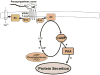
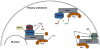
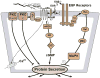
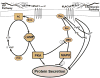
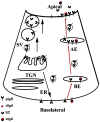
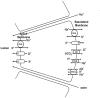
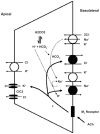
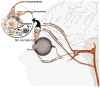
The lacrimal gland is the major contributor to the aqueous layer of the tear film which consists of water, electrolytes and proteins. The amount and composition of this layer is critical for the health, maintenance, and protection of the cells of the cornea and conjunctiva (the ocular surface). Small changes in the concentration of tear electrolytes have been correlated with dry eye syndrome. While the mechanisms of secretion of water, electrolytes and proteins from the lacrimal gland differ, all three are under tight neural control. This allows for a rapid response to meet the needs of the cells of the ocular surface in response to environmental conditions. The neural response consists of the activation of the afferent sensory nerves in the cornea and conjunctiva to stimulate efferent parasympathetic and sympathetic nerves that innervate the lacrimal gland. Neurotransmitters are released from the stimulated parasympathetic and sympathetic nerves that cause secretion of water, electrolytes, and proteins from the lacrimal gland and onto the ocular surface. This review focuses on the neural regulation of lacrimal gland secretion under normal and dry eye conditions.
Figures


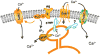
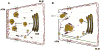
References
-
- Acosta MC, Peral A, Luna C, Pintor J, Belmonte C, Gallar J. Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers. Invest Ophthalmol Vis Sci. 2004;45(7):2333–2336. - PubMed
-
- Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001;42(9):2063–2067. - PubMed
-
- Allansmith MR, Kajiyama G, Abelson MB, Simon MA. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976;82(6):819–826. - PubMed
-
- Andersson SV, Hamm-Alvarez SF, Gierow JP. Integrin adhesion in regulation of lacrimal gland acinar cell secretion. Exp Eye Res. 2006;83(3):543–553. - PubMed
-
- Arslan G, Fredholm BB. Stimulatory and inhibitory effects of adenosine A(2A) receptors on nerve growth factor-induced phosphorylation of extracellular regulated kinases 1/2 in PC12 cells. Neurosci Lett. 2000;292(3):183–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

