Mitochondrial dysfunction contributes to impaired insulin secretion in INS-1 cells with dominant-negative mutations of HNF-1alpha and in HNF-1alpha-deficient islets
- PMID: 19376774
- PMCID: PMC2719317
- DOI: 10.1074/jbc.M807723200
Mitochondrial dysfunction contributes to impaired insulin secretion in INS-1 cells with dominant-negative mutations of HNF-1alpha and in HNF-1alpha-deficient islets
Abstract
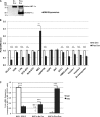
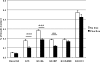
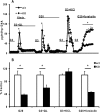
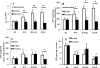
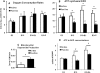
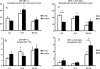
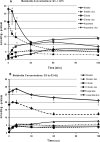
Maturity Onset Diabetes of the Young-type 3 (MODY-3) has been linked to mutations in the transcription factor hepatic nuclear factor (HNF)-1alpha, resulting in deficiency in glucose-stimulated insulin secretion. In INS-1 cells overexpressing doxycycline-inducible HNF-1alpha dominant-negative (DN-) gene mutations, and islets from Hnf-1alpha knock-out mice, insulin secretion was impaired in response to glucose (15 mm) and other nutrient secretagogues. Decreased rates of insulin secretion in response to glutamine plus leucine and to methyl pyruvate, but not potassium depolarization, indicate defects specific to mitochondrial metabolism. To identify the biochemical mechanisms responsible for impaired insulin secretion, we used (31)P NMR measured mitochondrial ATP synthesis (distinct from glycolytic ATP synthesis) together with oxygen consumption measurements to determine the efficiency of mitochondrial oxidative phosphorylation. Mitochondrial uncoupling was significantly higher in DN-HNF-1alpha cells, such that rates of ATP synthesis were decreased by approximately one-half in response to the secretagogues glucose, glutamine plus leucine, or pyruvate. In addition to closure of the ATP-sensitive K(+) channels with mitochondrial ATP synthesis, mitochondrial production of second messengers through increased anaplerotic flux has been shown to be critical for coupling metabolism to insulin secretion. (13)C-Isotopomer analysis and tandem mass spectrometry measurement of Krebs cycle intermediates revealed a negative impact of DN-HNF-1alpha and Hnf-1alpha knock-out on mitochondrial second messenger production with glucose but not amino acids. Taken together, these results indicate that, in addition to reduced glycolytic flux, uncoupling of mitochondrial oxidative phosphorylation contributes to impaired nutrient-stimulated insulin secretion with either mutations or loss of HNF-1alpha.
Figures
References
-
- Fajans S. S. ( 1989) Diabetes Metab. Rev. 5, 579– 606 - PubMed
-
- Yamagata K., Oda N., Kaisaki P. J., Menzel S., Furuta H., Vaxillaire M., Southam L., Cox R. D., Lathrop G. M., Boriraj V. V., Chen X., Cox N. J., Oda Y., Yano H., Le Beau M. M., Yamada S., Nishigori H., Takeda J., Fajans S. S., Hattersley A. T., Iwasaki N., Hansen T., Pederson O., Polonsky K. S., Bell G. I., et al. ( 1996) Nature 384, 455– 458 - PubMed
-
- Dukes I. D., Sreenan S., Roe M. W., Levisetti M., Zhou Y. P., Ostrega D., Bell G. I., Pontoglio M., Yaniv M., Philipson L., Polonsky K. S. ( 1998) J. Biol. Chem. 273, 24457– 24464 - PubMed
-
- Shih D. Q., Screenan S., Munoz K. N., Philipson L., Pontoglio M., Yaniv M., Polonsky K. S., Stoffel M. ( 2001) Diabetes 50, 2472– 2480 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

