Agrin regulation of alpha3 sodium-potassium ATPase activity modulates cardiac myocyte contraction
- PMID: 19376779
- PMCID: PMC2719333
- DOI: 10.1074/jbc.M806855200
Agrin regulation of alpha3 sodium-potassium ATPase activity modulates cardiac myocyte contraction
Abstract
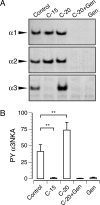
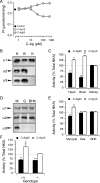
Drugs that inhibit Na,K-ATPases, such as digoxin and ouabain, alter cardiac myocyte contractility. We recently demonstrated that agrin, a protein first identified at the vertebrate neuromuscular junction, binds to and regulates the activity of alpha3 subunit-containing isoforms of the Na,K-ATPase in the mammalian brain. Both agrin and the alpha3 Na,K-ATPase are expressed in heart, but their potential for interaction and effect on cardiac myocyte function was unknown. Here we show that agrin binds to the alpha3 subunit of the Na,K-ATPase in cardiac myocyte membranes, inducing tyrosine phosphorylation and inhibiting activity of the pump. Agrin also triggers a rapid increase in cytoplasmic Na(+) in cardiac myocytes, suggesting a role in cardiac myocyte function. Consistent with this hypothesis, spontaneous contraction frequencies of cultured cardiac myocytes prepared from mice in which agrin expression is blocked by mutation of the Agrn gene are significantly higher than in the wild type. The Agrn mutant phenotype is rescued by acute treatment with recombinant agrin. Furthermore, exposure of wild type myocytes to an agrin antagonist phenocopies the Agrn mutation. These data demonstrate that the basal frequency of myocyte contraction depends on endogenous agrin-alpha3 Na,K-ATPase interaction and suggest that agrin modulation of the alpha3 Na,K-ATPase is important in regulating heart function.
Figures


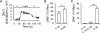

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

