Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway
- PMID: 19376810
- PMCID: PMC2716099
- DOI: 10.1152/ajpheart.00090.2009
Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway
Abstract
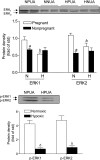
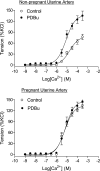
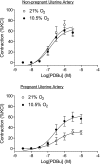
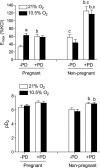
Chronic hypoxia during pregnancy has profound effects on uterine artery (UA) contractility and attenuates uterine blood flow. The present study tested the hypothesis that chronic hypoxia inhibits the pregnancy-induced reduction in pressure-dependent myogenic tone of resistance-sized UAs. UAs were isolated from nonpregnant ewes (NPUAs) and near-term pregnant ewes (PUAs) that had been maintained at sea level (approximately 300 m) or at high altitude (3,801 m) for 110 days. In normoxic animals, the pressure-dependent myogenic response was significantly attenuated in PUAs compared with NPUAs. Hypoxia significantly increased myogenic tone in PUAs and abolished its difference between PUAs and NPUAs. Consistently, there was a significant increase in PKC-mediated baseline Ca(2+) sensitivity of PUAs in hypoxic animals. Hypoxia significantly increased phorbol 12,13-dibutyrate (PDBu)-induced contractions in PUAs but not in NPUAs. Whereas the inhibition of ERK1/2 by PD-98059 potentiated PDBu-mediated contractions of PUAs in normoxic animals, it failed to do so in hypoxic animals. Hypoxia decreased ERK1/2 expression in PUAs. PDBu induced membrane translocation of PKC-alpha and PKC-epsilon. Whereas there were no significant differences in PKC-alpha translocation among all groups, the translocation of PKC-epsilon was significantly enhanced in NPUAs compared with PUAs in normoxic animals, and hypoxia significantly increased PKC-epsilon translocation in PUAs. In the presence of PD-98059, there were no significant differences in PDBu-induced PKC-epsilon translocation among all groups. Treatment of PUAs isolated from normoxic animals with 10.5% O(2) for 48 h ex vivo significantly increased PDBu-induced contractions and eliminated its difference between PUAs and NPUAs. The results suggest that hypoxia upregulates pressure-dependent myogenic tone through its direct effect in suppressing ERK1/2 activity and increasing the PKC signal pathway, leading to an increase in the Ca(2+) sensitivity of the myogenic mechanism in the UA during pregnancy.
Figures





References
-
- Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343: 651–653, 1990. - PubMed
-
- Bakker EN, Kerkhof CJ, Sipkema P. Signal transduction in spontaneous myogenic tone in isolated arterioles from rat skeletal muscle. Cardiovasc Res 41: 229–236, 1999. - PubMed
-
- Bialecki RA, Fisher CS, Murdoch WW, Barthlow HG. Chronic hypoxia increases staurosporine sensitivity of pulmonary artery smooth muscle to endothelin-1. Pulm Pharmacol Ther 11: 159–163, 1998. - PubMed
-
- Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008. - PubMed
-
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

