Role of the extracytoplasmic function sigma factor RpoE4 in oxidative and osmotic stress responses in Rhizobium etli
- PMID: 19376852
- PMCID: PMC2698498
- DOI: 10.1128/JB.01626-08
Role of the extracytoplasmic function sigma factor RpoE4 in oxidative and osmotic stress responses in Rhizobium etli
Abstract
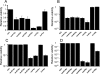
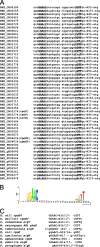
The aims of this study were to functionally characterize and analyze the transcriptional regulation and transcriptome of the Rhizobium etli rpoE4 gene. An R. etli rpoE4 mutant was sensitive to oxidative, saline, and osmotic stresses. Using transcriptional fusions, we determined that RpoE4 controls its own transcription and that it is negatively regulated by rseF (regulator of sigma rpoE4; CH03274), which is cotranscribed with rpoE4. rpoE4 expression was induced not only after oxidative, saline, and osmotic shocks, but also under microaerobic and stationary-phase growth conditions. The transcriptome analyses of an rpoE4 mutant and an rpoE4-overexpressing strain revealed that the RpoE4 extracytoplasmic function sigma factor regulates about 98 genes; 50 of them have the rpoE4 promoter motifs in the upstream regulatory regions. Interestingly, 16 of 38 genes upregulated in the rpoE4-overexpressing strain encode unknown putative cell envelope proteins. Other genes controlled by RpoE4 include rpoH2, CH00462, CH02434, CH03474, and xthA1, which encode proteins involved in the stress response (a heat shock sigma factor, a putative Mn-catalase, an alkylation DNA repair protein, pyridoxine phosphate oxidase, and exonuclease III, respectively), as well as several genes, such as CH01253, CH03555, and PF00247, encoding putative proteins involved in cell envelope biogenesis (a putative peptidoglycan binding protein, a cell wall degradation protein, and phospholipase D, respectively). These results suggest that rpoE4 has a relevant function in cell envelope biogenesis and that it plays a role as a general regulator in the responses to several kinds of stress.
Figures

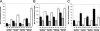
References
-
- Akbar, S., S. Lee, S. Boylan, and C. Price. 1999. Two genes from Bacillus subtilis under the sole control of the general stress transcription factor sigma B. Microbiology 1451069-1078. - PubMed
-
- Alba, B. M., and C. A. Gross. 2004. Regulation of the Escherichia coli σE-dependent envelope stress response. Mol. Microbiol. 52613-619. - PubMed
-
- Alvarez-Martinez, C. E., R. F. Lourenço, R. L. Baldini, M. T. Laub, and S. L. Gomes. 2007. The ECF sigma factor σT is involved in osmotic and oxidative stress responses in Caulobacter crescentus. Mol. Microbiol. 661240-1255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

