Enhancing the production of hydroxyl radicals by Pleurotus eryngii via quinone redox cycling for pollutant removal
- PMID: 19376890
- PMCID: PMC2698368
- DOI: 10.1128/AEM.02138-08
Enhancing the production of hydroxyl radicals by Pleurotus eryngii via quinone redox cycling for pollutant removal
Abstract
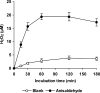
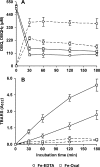
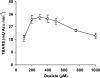
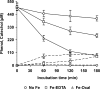
The induction of hydroxyl radical (OH) production via quinone redox cycling in white-rot fungi was investigated to improve pollutant degradation. In particular, we examined the influence of 4-methoxybenzaldehyde (anisaldehyde), Mn(2+), and oxalate on Pleurotus eryngii OH generation. Our standard quinone redox cycling conditions combined mycelium from laccase-producing cultures with 2,6-dimethoxy-1,4-benzoquinone (DBQ) and Fe(3+)-EDTA. The main reactions involved in OH production under these conditions have been shown to be (i) DBQ reduction to hydroquinone (DBQH(2)) by cell-bound dehydrogenase activities; (ii) DBQH(2) oxidation to semiquinone (DBQ(-)) by laccase; (iii) DBQ(-) autoxidation, catalyzed by Fe(3+)-EDTA, producing superoxide (O(2)(-)) and Fe(2+)-EDTA; (iv) O(2)(-) dismutation, generating H(2)O(2); and (v) the Fenton reaction. Compared to standard quinone redox cycling conditions, OH production was increased 1.2- and 3.0-fold by the presence of anisaldehyde and Mn(2+), respectively, and 3.1-fold by substituting Fe(3+)-EDTA with Fe(3+)-oxalate. A 6.3-fold increase was obtained by combining Mn(2+) and Fe(3+)-oxalate. These increases were due to enhanced production of H(2)O(2) via anisaldehyde redox cycling and O(2)(-) reduction by Mn(2+). They were also caused by the acceleration of the DBQ redox cycle as a consequence of DBQH(2) oxidation by both Fe(3+)-oxalate and the Mn(3+) generated during O(2)(-) reduction. Finally, induction of OH production through quinone redox cycling enabled P. eryngii to oxidize phenol and the dye reactive black 5, obtaining a high correlation between the rates of OH production and pollutant oxidation.
Figures







Similar articles
-
Induction of Extracellular Hydroxyl Radicals Production in the White-Rot Fungus Pleurotus eryngii for Dyes Degradation: An Advanced Bio-oxidation Process.J Fungi (Basel). 2024 Jan 7;10(1):52. doi: 10.3390/jof10010052. J Fungi (Basel). 2024. PMID: 38248961 Free PMC article.
-
Induction of extracellular hydroxyl radical production by white-rot fungi through quinone redox cycling.Appl Environ Microbiol. 2009 Jun;75(12):3944-53. doi: 10.1128/AEM.02137-08. Epub 2009 Apr 17. Appl Environ Microbiol. 2009. PMID: 19376892 Free PMC article.
-
Oxygen activation during oxidation of methoxyhydroquinones by laccase from Pleurotus eryngii.Appl Environ Microbiol. 2000 Jan;66(1):170-5. doi: 10.1128/AEM.66.1.170-175.2000. Appl Environ Microbiol. 2000. PMID: 10618219 Free PMC article.
-
Advanced oxidation of benzene, toluene, ethylbenzene and xylene isomers (BTEX) by Trametes versicolor.J Hazard Mater. 2010 Sep 15;181(1-3):181-6. doi: 10.1016/j.jhazmat.2010.04.114. J Hazard Mater. 2010. PMID: 20627409
-
Tire-rubber related pollutant 6-PPD quinone: A review of its transformation, environmental distribution, bioavailability, and toxicity.J Hazard Mater. 2023 Oct 5;459:132265. doi: 10.1016/j.jhazmat.2023.132265. Epub 2023 Aug 15. J Hazard Mater. 2023. PMID: 37595463 Review.
Cited by
-
The potential of fungi in the bioremediation of pharmaceutically active compounds: a comprehensive review.Front Microbiol. 2023 Jul 12;14:1207792. doi: 10.3389/fmicb.2023.1207792. eCollection 2023. Front Microbiol. 2023. PMID: 37502403 Free PMC article. Review.
-
Expanding the Physiological Role of Aryl-Alcohol Flavooxidases as Quinone Reductases.Appl Environ Microbiol. 2023 May 31;89(5):e0184422. doi: 10.1128/aem.01844-22. Epub 2023 May 8. Appl Environ Microbiol. 2023. PMID: 37154753 Free PMC article.
-
Removal of pharmaceutical compounds from urban wastewater by an advanced bio-oxidation process based on fungi Trametes versicolor immobilized in a continuous RBC system.Environ Sci Pollut Res Int. 2018 Dec;25(35):34884-34892. doi: 10.1007/s11356-017-1053-4. Epub 2017 Dec 20. Environ Sci Pollut Res Int. 2018. PMID: 29264858
-
The Pseudomonas ligninolytic catalytic network reveals the importance of auxiliary enzymes in lignin biocatalysts.Proc Natl Acad Sci U S A. 2025 Jan 28;122(4):e2417343122. doi: 10.1073/pnas.2417343122. Epub 2025 Jan 24. Proc Natl Acad Sci U S A. 2025. PMID: 39854233 Free PMC article.
-
Induction of Extracellular Hydroxyl Radicals Production in the White-Rot Fungus Pleurotus eryngii for Dyes Degradation: An Advanced Bio-oxidation Process.J Fungi (Basel). 2024 Jan 7;10(1):52. doi: 10.3390/jof10010052. J Fungi (Basel). 2024. PMID: 38248961 Free PMC article.
References
-
- Alnaizy, R., and A. Akgerman. 2000. Advanced oxidation of phenolic compounds. Adv. Environ. Res. 4:233-244.
-
- Backa, S., J. Gierer, T. Reitberger, and T. Nilsson. 1993. Hydroxyl radical activity associated with the growth of white-rot fungi. Holzforschung 47:181-187.
-
- Cameron, M. D., and S. D. Aust. 1999. Degradation of chemicals by reactive radicals produced by cellobiose dehydrogenase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 367:115-121. - PubMed
-
- Constam, D., A. Muheim, W. Zimmermann, and A. Fiechter. 1991. Purification and partial characterization of an intracellular NADH:quinone oxidoreductase from Phanerochaete chrysosporium. J. Gen. Microbiol. 137:2209-2214.
-
- Glaze, W. H., J. W. Kang, and D. H. Chapin. 1987. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 9:335-352.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

