Misexpression of FATTY ACID ELONGATION1 in the Arabidopsis epidermis induces cell death and suggests a critical role for phospholipase A2 in this process
- PMID: 19376931
- PMCID: PMC2685613
- DOI: 10.1105/tpc.109.065565
Misexpression of FATTY ACID ELONGATION1 in the Arabidopsis epidermis induces cell death and suggests a critical role for phospholipase A2 in this process
Abstract
Very-long-chain fatty acids (VLCFAs) are important functional components of various lipid classes, including cuticular lipids in the higher plant epidermis and lipid-derived second messengers. Here, we report the characterization of transgenic Arabidopsis thaliana plants that epidermally express FATTY ACID ELONGATION1 (FAE1), the seed-specific beta-ketoacyl-CoA synthase (KCS) catalyzing the first rate-limiting step in VLCFA biosynthesis. Misexpression of FAE1 changes the VLCFAs in different classes of lipids but surprisingly does not complement the KCS fiddlehead mutant. FAE1 misexpression plants are similar to the wild type but display an essentially glabrous phenotype, owing to the selective death of trichome cells. This cell death is accompanied by membrane damage, generation of reactive oxygen species, and callose deposition. We found that nuclei of arrested trichome cells in FAE1 misexpression plants cell-autonomously accumulate high levels of DNA damage, including double-strand breaks characteristic of lipoapoptosis. A chemical genetic screen revealed that inhibitors of KCS and phospholipase A2 (PLA2), but not inhibitors of de novo ceramide biosynthesis, rescue trichome cells from death. These results support the functional role of acyl chain length of fatty acids and PLA2 as determinants for programmed cell death, likely involving the exchange of VLCFAs between phospholipids and the acyl-CoA pool.
Figures


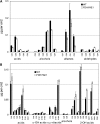


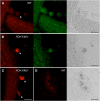



Comment in
-
Lipid determinants of cell death.Plant Signal Behav. 2009 Jul;4(7):625-8. doi: 10.1105/tpc.109.065565. Epub 2009 Jul 4. Plant Signal Behav. 2009. PMID: 19820339 Free PMC article.
References
-
- Adam, L., and Somerville, S.C. (1996). Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 9 341–356. - PubMed
-
- Bach, L., Michaelson, L.V., Haslam, R., Bellec, Y., Gissot, L., Marion, J., Da Costa, M., Boutin, J.P., Miquel, M., and Tellier, F. (2008). The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc. Natl. Acad. Sci. USA 105 14727–14731. - PMC - PubMed
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In-planta Agrobacterium-mediated gene-transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris Life Sci. 316 1194–1199.
-
- Blacklock, B.J., and Jaworski, J.G. (2006). Substrate specificity of Arabidopsis 3-ketoacyl-CoA synthases. Biochem. Biophys. Res. Commun. 346 583–590. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

