Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis)
- PMID: 19376944
- PMCID: PMC2726848
- DOI: 10.1242/jeb.023945
Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis)
Abstract
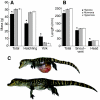
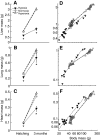
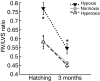
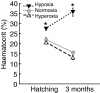
Recent palaeoatmospheric models suggest large-scale fluctuations in ambient oxygen level over the past 550 million years. To better understand how global hypoxia and hyperoxia might have affected the growth and physiology of contemporary vertebrates, we incubated eggs and raised hatchlings of the American alligator. Crocodilians are one of few vertebrate taxa that survived these global changes with distinctly conservative morphology. We maintained animals at 30 degrees C under chronic hypoxia (12% O(2)), normoxia (21% O(2)) or hyperoxia (30% O(2)). At hatching, hypoxic animals were significantly smaller than their normoxic and hyperoxic siblings. Over the course of 3 months, post-hatching growth was fastest under hyperoxia and slowest under hypoxia. Hypoxia, but not hyperoxia, caused distinct scaling of major visceral organs-reduction of liver mass, enlargement of the heart and accelerated growth of lungs. When absorptive and post-absorptive metabolic rates were measured in juvenile alligators, the increase in oxygen consumption rate due to digestion/absorption of food was greatest in hyperoxic alligators and smallest in hypoxic ones. Hyperoxic alligators exhibited the lowest breathing rate and highest oxygen consumption per breath. We suggest that, despite compensatory cardiopulmonary remodelling, growth of hypoxic alligators is constrained by low atmospheric oxygen supply, which may limit their food utilisation capacity. Conversely, the combination of elevated metabolism and low cost of breathing in hyperoxic alligators allows for a greater proportion of metabolised energy to be available for growth. This suggests that growth and metabolic patterns of extinct vertebrates would have been significantly affected by changes in the atmospheric oxygen level.
Figures
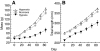
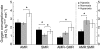
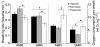
References
-
- Andrade, D. V. and Abe, A. S. (1999). Gas exchange and ventilation during dormancy in the tegu lizard Tupinambis merianae. J. Exp. Biol. 202, 3677-3685. - PubMed
-
- Andrews, R. M. (2001). Low oxygen: a constraint on the evolution of viviparity in reptiles. Physiol. Biochem. Zool. 75, 145-154. - PubMed
-
- Bartlett, D., Jr and Remmers, J. E. (1971). Effects of high altitude exposure on the lungs of young rats. Respir. Physiol. 13, 116-125. - PubMed
-
- Bavis, R. W. (2005). Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir. Physiol. Neurobiol. 149, 287-299. - PubMed
-
- Bergman, N. M., Lenton, T. M. and Watson, A. J. (2004). COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 304, 397-437.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

