Functional and structural characterization of a dense core secretory granule sorting domain from the PC1/3 protease
- PMID: 19376969
- PMCID: PMC2678656
- DOI: 10.1073/pnas.0809576106
Functional and structural characterization of a dense core secretory granule sorting domain from the PC1/3 protease
Abstract
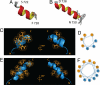
Several peptide hormones are initially synthesized as inactive precursors. It is only on entry of these prohormones and their processing proteases into dense core secretory granules (DCSGs) that the precursors are cleaved to generate their active forms. Prohormone convertase (PC)1/3 is a processing protease that is targeted to DCSGs. The signal for targeting PC1/3 to DCSGs resides in its carboxy-terminal tail (PC1/3(617-753)), where 3 regions (PC1/3(617-625), PC1/3(665-682), and PC1/3(711-753)) are known to aid in sorting and membrane association. In this article, we have determined a high-resolution structure of the extreme carboxy-terminal sorting domain, PC1/3(711-753) in micelles by NMR spectroscopy. PC1/3(711-753) contains 2 alpha helices located between residues 722-728 and 738-750. Functional assays demonstrate that the second helix (PC1/3(738-750)) is necessary and sufficient to target a constitutively secreted protein to granules, and that L(745) anchors a hydrophobic patch that is critical for sorting. Also, we demonstrate that calcium binding by the second helix of PC1/3(711-753) promotes aggregation of the domain via the hydrophobic patch centered on L(745). These results provide a structure-function analysis of a DCSG-sorting domain, and reveal the importance of a hydrophobic patch and calcium binding in controlling the sorting of proteins containing alpha helices to DCSGs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Taugner R, Kim SJ, Murakami K, Waldherr R. The fate of prorenin during granulopoiesis in epithelioid cells—Immunocytochemical experiments with antisera against renin and different portions of the prorenin prosegment. Histochemistry. 1987;86:249–253. - PubMed
-
- Meldolesi J, Chieregatti E, Malosio ML. Requirements for the identification of dense-core granules. Trends Cell Biol. 2004;14:13–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

