Therapy with mycophenolate mofetil for refractory acute and chronic GVHD
- PMID: 19377515
- PMCID: PMC2791193
- DOI: 10.1038/bmt.2009.76
Therapy with mycophenolate mofetil for refractory acute and chronic GVHD
Abstract
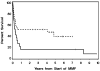
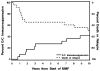
We evaluated the pharmacokinetics and efficacy of oral mycophenolate mofetil (MMF) for treatment of refractory GVHD. In a prospective study of acute GVHD, 9 of 19 patients (47%) had a response and 10 (53%) had no improvement. Survival at 6 and 12 months after the start of MMF was 37 and 16%, respectively. In a retrospective study of acute GVHD, 14 of 29 patients (48%) had a response and 15 (52%) had no improvement. Survival at 6 and 12 months was 55 and 52%, respectively. In a prospective study of chronic GVHD, the cumulative incidence of disease resolution and withdrawal of all systemic immunosuppressive treatment was 9, 17 and 26% at 12, 24 and 36 months, respectively, after starting MMF. Thirteen patients (59%) required additional systemic immunosuppressive treatment for chronic GVHD. Nine of the 42 patients (21%) in the prospective studies discontinued MMF treatment because of toxicity. The area under the curve plasma concentrations of mycophenolic acid seemed to be suboptimal among patients with acute GVHD but not among those with chronic GVHD. MMF can be used effectively for treatment of GVHD.
Figures
Similar articles
-
Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant.Eur J Haematol. 2004 Jul;73(1):56-61. doi: 10.1111/j.1600-0609.2004.00247.x. Eur J Haematol. 2004. PMID: 15182339 Clinical Trial.
-
A Pilot Study of Continuous Infusion of Mycophenolate Mofetil for Prophylaxis of Graft-versus-Host-Disease in Pediatric Patients.Biol Blood Marrow Transplant. 2016 Apr;22(4):682-689. doi: 10.1016/j.bbmt.2015.12.013. Epub 2015 Dec 29. Biol Blood Marrow Transplant. 2016. PMID: 26740371
-
Mycophenolate mofetil for treatment of steroid-refractory acute graft-versus-host disease after pediatric hematopoietic stem cell transplantation.Pediatr Transplant. 2015 Sep;19(6):652-8. doi: 10.1111/petr.12545. Epub 2015 Jun 23. Pediatr Transplant. 2015. PMID: 26103520
-
Mycophenolate mofetil for the prevention and treatment of graft-versus-host disease following stem cell transplantation: preliminary findings.Bone Marrow Transplant. 2001 Jun;27(12):1255-62. doi: 10.1038/sj.bmt.1703076. Bone Marrow Transplant. 2001. PMID: 11548843 Review.
-
Mycophenolate mofetil: fully utilizing its benefits for GvHD prophylaxis.Int J Hematol. 2012 Jul;96(1):10-25. doi: 10.1007/s12185-012-1086-x. Epub 2012 May 17. Int J Hematol. 2012. PMID: 22592321 Review.
Cited by
-
Cost-Effectiveness of Extracorporeal Photopheresis in Patients With Chronic Graft-vs-Host Disease.J Health Econ Outcomes Res. 2024 Feb 1;11(1):23-31. doi: 10.36469/001c.92028. eCollection 2024. J Health Econ Outcomes Res. 2024. PMID: 38312919 Free PMC article.
-
New and emerging therapies for acute and chronic graft versus host disease.Ther Adv Hematol. 2018 Jan;9(1):21-46. doi: 10.1177/2040620717741860. Epub 2017 Nov 28. Ther Adv Hematol. 2018. PMID: 29317998 Free PMC article. Review.
-
Mesenchymal stromal cells plus basiliximab improve the response of steroid-refractory acute graft-versus-host disease as a second-line therapy: a multicentre, randomized, controlled trial.BMC Med. 2024 Feb 27;22(1):85. doi: 10.1186/s12916-024-03275-5. BMC Med. 2024. PMID: 38413930 Free PMC article. Clinical Trial.
-
Modern-Era Challenges in the Clinical Management of Graft-Versus-Host Disease.Adv Exp Med Biol. 2025;1475:103-128. doi: 10.1007/978-3-031-84988-6_6. Adv Exp Med Biol. 2025. PMID: 40488826 Review.
-
Secondary treatment of acute graft-versus-host disease: a critical review.Biol Blood Marrow Transplant. 2012 Jul;18(7):982-8. doi: 10.1016/j.bbmt.2012.04.006. Epub 2012 Apr 14. Biol Blood Marrow Transplant. 2012. PMID: 22510383 Free PMC article. Review.
References
-
- Sullivan KM. Graft-versus-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. 3. Blackwell Publishing Ltd; Malden, MA: 2004. pp. 635–664.
-
- Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. - PubMed
-
- Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. - PubMed
-
- Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. - PubMed
-
- Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328:593–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources