Recent advances in structure-functional studies of mitochondrial factor B
- PMID: 19377834
- PMCID: PMC2880480
- DOI: 10.1007/s10863-009-9210-1
Recent advances in structure-functional studies of mitochondrial factor B
Abstract
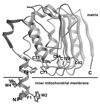
Since the early studies on the resolution and reconstitution of the oxidative phosphorylation system from animal mitochondria, coupling factor B was recognized as an essential component of the machinery responsible for energy-driven ATP synthesis. At the phenomenological level, factor B was agreed to lie at the interface of energy transfer between the respiratory chain and the ATP synthase complex. However, biochemical characterization of the factor B polypeptide has proved difficult. It was not until 1990 that the N-terminal amino acid sequence of bovine mitochondrial factor B was reported, which followed, a decade later, by the report describing the amino acid sequence of full-length human factor B and its functional characterization. The present review summarizes the recent advances in structure-functional studies of factor B, including its recently determined crystal structure at 0.96 A resolution. Ectopic expression of human factor B in cultured animal cells has unexpectedly revealed its role in shaping mitochondrial morphology. The supramolecular assembly of ATP synthase as dimer ribbons at highly curved apices of the mitochondrial cristae was recently suggested to optimize ATP synthesis under proton-limited conditions. We propose that the binding of the ATP synthase dimers with factor B tetramers could be a means to enhance the efficiency of the terminal step of oxidative phosphorylation in animal mitochondria.
Figures
Similar articles
-
Crystal structure of bovine mitochondrial factor B at 0.96-A resolution.Proc Natl Acad Sci U S A. 2008 Sep 9;105(36):13379-84. doi: 10.1073/pnas.0805689105. Epub 2008 Sep 3. Proc Natl Acad Sci U S A. 2008. PMID: 18768789 Free PMC article.
-
Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes.Proc Natl Acad Sci U S A. 2020 Feb 4;117(5):2412-2421. doi: 10.1073/pnas.1917968117. Epub 2020 Jan 21. Proc Natl Acad Sci U S A. 2020. PMID: 31964824 Free PMC article.
-
Coupling factor B affects the morphology of mitochondria.J Bioenerg Biomembr. 2010 Feb;42(1):29-35. doi: 10.1007/s10863-009-9263-1. Epub 2010 Jan 13. J Bioenerg Biomembr. 2010. PMID: 20069349 Free PMC article.
-
Who and how in the regulation of mitochondrial cristae shape and function.Biochem Biophys Res Commun. 2018 May 27;500(1):94-101. doi: 10.1016/j.bbrc.2017.04.088. Epub 2017 Apr 21. Biochem Biophys Res Commun. 2018. PMID: 28438601 Review.
-
Cryo-EM of ATP synthases.Curr Opin Struct Biol. 2018 Oct;52:71-79. doi: 10.1016/j.sbi.2018.08.005. Epub 2018 Sep 18. Curr Opin Struct Biol. 2018. PMID: 30240940 Review.
Cited by
-
Epigenetic regulation of the respiratory chain by a mitochondrial distress-related redox signal.Front Cell Dev Biol. 2025 Aug 5;13:1608400. doi: 10.3389/fcell.2025.1608400. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40837190 Free PMC article.
-
Regulation of mitochondrial ATP synthase in cardiac pathophysiology.Am J Cardiovasc Dis. 2015 Mar 20;5(1):19-32. eCollection 2015. Am J Cardiovasc Dis. 2015. PMID: 26064790 Free PMC article. Review.
-
Mitochondrial F-ATP Synthase and Its Transition into an Energy-Dissipating Molecular Machine.Oxid Med Cell Longev. 2019 Apr 15;2019:8743257. doi: 10.1155/2019/8743257. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31178976 Free PMC article. Review.
-
Cardiac mitochondrial matrix and respiratory complex protein phosphorylation.Am J Physiol Heart Circ Physiol. 2012 Oct 15;303(8):H940-66. doi: 10.1152/ajpheart.00077.2012. Epub 2012 Aug 10. Am J Physiol Heart Circ Physiol. 2012. PMID: 22886415 Free PMC article. Review.
-
The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology.Physiol Rev. 2015 Oct;95(4):1111-55. doi: 10.1152/physrev.00001.2015. Physiol Rev. 2015. PMID: 26269524 Free PMC article. Review.
References
-
- Angevine CM, Herold KA, Vincent OD, Fillingame RH. J Biol Chem. 2007;282:9001–9007. - PubMed
-
- Arselin G, Vaillier J, Salin B, Schaeffer J, Giraud MF, Dautant A, Brethes D, Velours J. J Biol Chem. 2004;279:40392–40399. - PubMed
-
- Belogrudov GI, Hatefi Y. J Biol Chem. 2002;277:6097–6103. - PubMed
-
- Belogrudov GI. Arch Biochem Biophys. 2002;406:271–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

