Imaging specific cell surface protease activity in living cells using reengineered bacterial cytotoxins
- PMID: 19377967
- PMCID: PMC2753202
- DOI: 10.1007/978-1-60327-003-8_7
Imaging specific cell surface protease activity in living cells using reengineered bacterial cytotoxins
Abstract
The scarcity of methods to visualize the activity of individual cell surface proteases in situ has hampered basic research and drug development efforts. In this chapter, we describe a simple, sensitive, and noninvasive assay that uses nontoxic reengineered bacterial cytotoxins with altered protease cleavage specificity to visualize specific cell surface proteolytic activity in single living cells. The assay takes advantage of the absolute requirement for site-specific endoproteolytic cleavage of cell surface-bound anthrax toxin protective antigen for its capacity to translocate an anthrax toxin lethal factor-beta-lactamase fusion protein to the cytoplasm. A fluorogenic beta-lactamase substrate is then used to visualize the cytoplasmically translocated anthrax toxin lethal factor-beta-lactamase fusion protein. By using anthrax toxin protective antigen variants that are reengineered to be cleaved by furin, urokinase plasminogen activator, or metalloproteinases, the cell surface activities of each of these proteases can be specifically and quantitatively determined with single cell resolution. The imaging assay is excellently suited for fluorescence microscope, fluorescence plate reader, and flow cytometry formats, and it can be used for a variety of purposes.
Figures
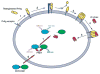

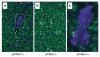

References
-
- Werb Z. ECM and cell surface pro-teolysis: regulating cellular ecology. Cell. 1997;91(4):439–42. - PubMed
-
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6(4):149–56. - PubMed
-
- Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477(12):98–111. - PubMed
-
- Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291(2):113–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

