Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity
- PMID: 19378257
- PMCID: PMC2759942
- DOI: 10.1387/ijdb.072564mp
Analyses of regenerative wave patterns in adult hair follicle populations reveal macro-environmental regulation of stem cell activity
Abstract
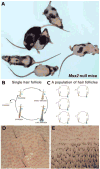
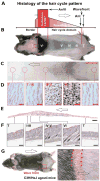
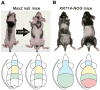
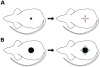
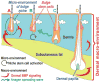
The control of hair growth in the adult mammalian coat is a fascinating topic which has just begun to be explored with molecular genetic tools. Complex hair cycle domains and regenerative hair waves are present in normal adult (> 2 month) mice, but more apparent in mutants with cyclic alopecia phenotypes. Each hair cycle domain consists of initiation site(s), a propagating wave and boundaries. By analyzing the dynamics of hair growth, time required for regeneration after plucking, in situ hybridization and reporter activity, we showed that there is oscillation of intra-follicular Wnt signaling which is synchronous with hair cycling, and there is oscillation of dermal bone morphogenetic protein (BMP) signaling which is asynchronous with hair cycling. The interactions of these two rhythms lead to the recognition of refractory and competent phases in the telogen, and autonomous and propagating phases in the anagen. Boundaries form when propagating anagen waves reach follicles which are in refractory telogen. Experiments showed that Krt14-Nog mice have shortened refractory telogen and simplified wave dynamics. Krt14-Nog skin grafts exhibit non-autonomous interactions with surrounding host skin. Implantation of BMP coated beads into competent telogen skin prevents hair wave propagation around the bead. Thus, we have developed a new molecular understanding of the classic early concepts of inhibitory "chalone", suggesting that stem cells within the hair follicle micro-environment, or other organs, are subject to a higher level of macro-environmental regulation. Such a novel understanding has important implications in the field of regenerative medicine. The unexpected links with Bmp2 expression in subcutaneous adipocytes has implications for systems biology and Evo-Devo.
Figures







Comment in
-
Pattern formation today.Int J Dev Biol. 2009;53(5-6):653-8. doi: 10.1387/ijdb.082594cc. Int J Dev Biol. 2009. PMID: 19557673 Free PMC article. Review.
References
-
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
-
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, Mcmahon JA, Peters C, Lauster R, Mcmahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. - PubMed
-
- Botchkarev VA, Botchkareva NV, Nakamura M, Huber O, Funa K, Lauster R, Paus R, Gilchrest BA. Noggin is required for induction of the hair follicle growth phase in postnatal skin. FASEB J. 2001;5:2205–2214. - PubMed
-
- Botchkarev VA, Botchkareva NV, Huber O, Funa K, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (non-tylotrich) hair follicles. J Invest Dermatol. 2002;118:3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

