Clinical applications of magnetoencephalography
- PMID: 19378272
- PMCID: PMC6870693
- DOI: 10.1002/hbm.20792
Clinical applications of magnetoencephalography
Abstract
Magnetoencephalography (MEG), in which magnetic fields generated by brain activity are recorded outside of the head, is now in routine clinical practice throughout the world. MEG has become a recognized and vital part of the presurgical evaluation of patients with epilepsy and patients with brain tumors. We review investigations that show an improvement in the postsurgical outcomes of patients with epilepsy by localizing epileptic discharges. We also describe the most common clinical MEG applications that affect the management of patients, and discuss some applications that are close to having a clinical impact on patients.
(c) 2009 Wiley-Liss, Inc.
Figures
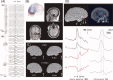
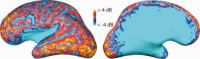
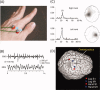
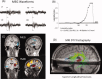

References
-
- Ahlfors SP,Simpson GV ( 2004): Geometrical interpretation of fMRI‐guided MEG/EEG inverse estimates. Neuroimage 22: 323–332. - PubMed
-
- Barkley GL ( 2004): Controversies in neurophysiology. MEG is superior to EEG in localization of interictal epileptiform activity: Pro. Clin Neurophysiol 115: 1001–1009. - PubMed
-
- Billingsley‐Marshall RL,Clear T,Mencl WE,Simos PG,Swank PR,Men D,Sarkari S,Castillo EM,Papanicolaou AC ( 2007): A comparison of functional MRI and magnetoencephalography for receptive language mapping. J Neurosci Methods 161: 306–313. - PubMed
-
- Dale AM,Liu AK,Fischl BR,Buckner RL,Belliveau JW,Lewine JD,Halgren E ( 2000): Dynamic statistical parametric mapping: Combining fMRI and MEG for high‐resolution imaging of cortical activity. Neuron 26: 55–67. - PubMed
-
- de Jongh A,de Munck JC,Goncalves SI,Ossenblok P ( 2005): Differences in MEG/EEG epileptic spike yields explained by regional differences in signal‐to‐noise ratios. J Clin Neurophysiol 22: 153–158. - PubMed

