A stereoselective synthesis of the bromopyrrole natural product (-)-agelastatin A
- PMID: 19378315
- PMCID: PMC2791535
- DOI: 10.1002/anie.200806292
A stereoselective synthesis of the bromopyrrole natural product (-)-agelastatin A
Abstract
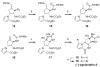
Weaving an intricate web: A stereoselective synthesis of (-)-agelastatin A has been developed, which requires 11 steps from commercially available starting material. The application of a Rh-catalyzed intramolecular olefin aziridination reaction and the subsequent manipulation of the resulting tricyclic intermediate (see scheme) punctuate this study.
Figures
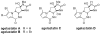
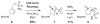
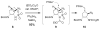
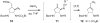
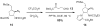
Similar articles
-
Total synthesis of (+/-)-agelastatin A, a potent inhibitor of osteopontin-mediated neoplastic transformations.Org Lett. 2009 Mar 19;11(6):1341-4. doi: 10.1021/ol900133v. Org Lett. 2009. PMID: 19228041 Free PMC article.
-
A stereodivergent strategy to both product enantiomers from the same enantiomer of a stereoinducing catalyst: agelastatin A.Chemistry. 2009 Jul 13;15(28):6910-9. doi: 10.1002/chem.200900794. Chemistry. 2009. PMID: 19533707 Free PMC article.
-
New class of nucleophiles for palladium-catalyzed asymmetric allylic alkylation. Total synthesis of agelastatin A.J Am Chem Soc. 2006 May 10;128(18):6054-5. doi: 10.1021/ja061105q. J Am Chem Soc. 2006. PMID: 16669672 Free PMC article.
-
Recent advances in natural product synthesis by using intramolecular Diels-Alder reactions.Chem Rev. 2005 Dec;105(12):4779-807. doi: 10.1021/cr040632u. Chem Rev. 2005. PMID: 16351062 Review. No abstract available.
-
Strategies towards the synthesis of calyciphylline A-type Daphniphyllum alkaloids.Nat Prod Rep. 2014 Apr;31(4):550-62. doi: 10.1039/c3np70115h. Epub 2014 Mar 5. Nat Prod Rep. 2014. PMID: 24595901 Review.
Cited by
-
Bioinspired total synthesis of agelastatin A.Angew Chem Int Ed Engl. 2012 Jul 9;51(28):6870-3. doi: 10.1002/anie.201200959. Epub 2012 Jun 11. Angew Chem Int Ed Engl. 2012. PMID: 22689447 Free PMC article.
-
Late-stage and strain-accelerated oxidation enabled synthesis of haouamine A.Chem Sci. 2020 Jun 24;11(31):8132-8137. doi: 10.1039/d0sc02299c. eCollection 2020 Aug 21. Chem Sci. 2020. PMID: 33033612 Free PMC article.
-
A submarine journey: the pyrrole-imidazole alkaloids.Mar Drugs. 2009 Nov 27;7(4):705-53. doi: 10.3390/md7040705. Mar Drugs. 2009. PMID: 20098608 Free PMC article. Review.
-
Syntheses of Cyclic Guanidine-Containing Natural Products.Tetrahedron. 2015 Feb 25;71(8):1145-1173. doi: 10.1016/j.tet.2014.11.056. Tetrahedron. 2015. PMID: 25684829 Free PMC article.
-
Biosynthesis, asymmetric synthesis, and pharmacology, including cellular targets, of the pyrrole-2-aminoimidazole marine alkaloids.Nat Prod Rep. 2011 Jul;28(7):1229-60. doi: 10.1039/c0np00013b. Epub 2011 May 9. Nat Prod Rep. 2011. PMID: 21556392 Free PMC article. Review.
References
-
-
For reviews on bromopyrrole natural products, see: Dembitsky VM. Russ Bioorg Chem. 2002;19:170–182.Jacquot DEN, Lindel T. Curr Org Chem. 2005;9:1551–1565.Weinreb SM. Nat Prod Rpts. 2007;24:931–948.Köck M, Grube A, Seiple AB, Baran PS. Angew Chem Int Ed. 2007;46:6586–6594.
-
-
- D’Ambrosio M, Guerriero A, Debitus C, Ribes O, Pusset J, Leroy S, Pietra F. J Chem Soc Chem Comm. 1993:1305–1306.
- D’Ambrosio M, Guerriero A, Chiasera G, Pietra F. Helv Chim Acta. 1994;77:1895–1902.
-
- Hong TW, Jimenez DR, Molinski TF. J Nat Prod. 1998;61:158–161. - PubMed
-
- D’Ambrosio M, Guerriero A, Ripamonti M, Debitus C, Waikedre J, Pietra F. Helv Chim Acta. 1996;79:727–735.
- Pettit GR, Ducki S, Herald DL, Doubek DL, Schmidt JM, Chapuis JC. Oncology Res. 2005;15:11–20. - PubMed
- Mason CK, McFarlane S, Johnston PG, Crowe P, Erwin PJ, Domostoj MM, Campbell FC, Manaviazar S, Hale KJ, El-Tanani M. Mol Cancer Ther. 2008;7:548–558. - PubMed
-
-
It has also been suggested that agelastatin A inhibits GSK-3β, an enzyme implicated in the development of the neurofibrillary tangles associated with Alzheimer’s disease, see: Meijer L, Thunnissen AMWH, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkow EM, Kim SH, Pettit GR. Chem Biol. 2000;7:51–63.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

