VMA21 deficiency: a case of myocyte indigestion
- PMID: 19379689
- PMCID: PMC2731491
- DOI: 10.1016/j.cell.2009.04.005
VMA21 deficiency: a case of myocyte indigestion
Abstract
The Vma21p protein in yeast is an essential assembly chaperone for the vacuolar ATPase, the major proton pump of cellular membranes. In this issue, Ramachandran et al. (2009) report that mutations in the gene encoding the human homolog VMA21 cause the disease X-linked myopathy with excessive autophagy through an unexpected mechanism.
Figures
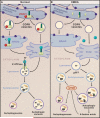
Comment on
-
VMA21 deficiency causes an autophagic myopathy by compromising V-ATPase activity and lysosomal acidification.Cell. 2009 Apr 17;137(2):235-46. doi: 10.1016/j.cell.2009.01.054. Cell. 2009. Retraction in: Cell. 2010 Sep 17;142(6):984. doi: 10.1016/j.cell.2010.08.034. PMID: 19379691 Retracted.
References
-
- Forgac M. Nat. Rev. Mol. Cell Biol. 2007;8:917–929. - PubMed
-
- Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Anders-son AK, Wallbrandt P, Zecca L, et al. Nat. Genet. 2000;25:343–346. - PubMed
-
- Kalimo H, Savontaus ML, Lang H, Paljarvi L, Sonninen V, Dean PB, Katevuo K, Salminen A. Ann. Neurol. 1988;23:258–265. - PubMed
-
- Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, et al. Nat. Genet. 1999;21:84–90. - PubMed
-
- Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, Budde B, Nurnberg P, Foulquier F, Lefeber D, et al. Nat. Genet. 2008;40:32–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

