Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site
- PMID: 19379698
- PMCID: PMC2807741
- DOI: 10.1016/j.cell.2009.02.018
Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site
Abstract
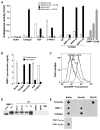
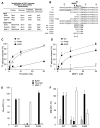
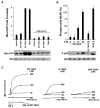
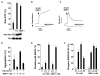
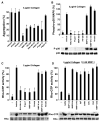
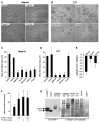
Matrix metalloproteases (MMPs) play important roles in normal and pathological remodeling processes including atherothrombotic disease, inflammation, angiogenesis, and cancer. MMPs have been viewed as matrix-degrading enzymes, but recent studies have shown that they possess direct signaling capabilities. Platelets harbor several MMPs that modulate hemostatic function and platelet survival; however their mode of action remains unknown. We show that platelet MMP-1 activates protease-activated receptor-1 (PAR1) on the surface of platelets. Exposure of platelets to fibrillar collagen converts the surface-bound proMMP-1 zymogen to active MMP-1, which promotes aggregation through PAR1. Unexpectedly, MMP-1 cleaves PAR1 at a distinct site that strongly activates Rho-GTP pathways, cell shape change and motility, and MAPK signaling. Blockade of MMP1-PAR1 curtails thrombogenesis under arterial flow conditions and inhibits thrombosis in animals. These studies provide a link between matrix-dependent activation of metalloproteases and platelet-G protein signaling and identify MMP1-PAR1 as a potential target for the prevention of arterial thrombosis.
Figures
References
-
- Bergmeier W, Burger PC, Piffath CL, Hoffmeister KM, Hartwig JH, Nieswandt B, Wagner DD. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 2003;102:4229–4235. - PubMed
-
- Berman J, Green M, Sugg E, Anderegg R, Millington DS, Norwood DL, McGeehan J, Wiseman J. Rapid optimization of enzyme substrates using defined substrate mixtures. J Biol Chem. 1992;267:1434–1437. - PubMed
-
- Bhatt DL, Topol EJ. Scientific and Therapeutic Advances in Antiplatelet Therapy. Nature Rev Drug Discovery. 2003;2:15–28. - PubMed
-
- Boire A, Covic L, Agarwal A, Jacques S, Sharifi S, Kuliopulos A. PAR1 is a Matrix Metalloprotease-1 Receptor that Promotes Invasion and Tumorigenesis of Breast Cancer Cells. Cell. 2005;120:303–313. - PubMed
-
- Chang JY. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur J Biochem. 1985;151:217–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

