Molecular mechanisms controlling E-cadherin expression in breast cancer
- PMID: 19379710
- PMCID: PMC2700729
- DOI: 10.1016/j.bbrc.2009.04.051
Molecular mechanisms controlling E-cadherin expression in breast cancer
Abstract
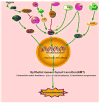
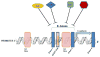
Disruption of cell-cell adhesion, which is essential for the maintenance of epithelial plasticity and is mediated by a class of proteins called cadherins, is an initial event in the progression of cancer. Cadherins are Ca(2+)-dependent transmembrane proteins that are associated with actin via other cytoplasmic proteins. Disruption of cell-cell adhesion during cancer progression is an important event during cancer initiation and metastasis. E-cadherin, one of the most widely studied tumor suppressors in breast cancer, belongs to a family of calcium-dependent cell adhesion molecules. Various signaling molecules and transcription factors regulate the expression of E-cadherin. Loss of E-cadherin has been reported to induce epithelial-mesenchymal transition in several cancers. This review highlights recent advances in defining the mechanisms that regulate E-cadherin expression in breast cancer.
Figures


References
-
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. - PubMed
-
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. - PubMed
-
- Heimann R, Hellman S. Clinical progression of breast cancer malignant behavior: what to expect and when to expect it. J Clin Oncol. 2000;18:591–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

