Ca2+-dependent photocrosslinking of tropomyosin residue 146 to residues 157-163 in the C-terminal domain of troponin I in reconstituted skeletal muscle thin filaments
- PMID: 19379756
- PMCID: PMC2805953
- DOI: 10.1016/j.jmb.2009.04.027
Ca2+-dependent photocrosslinking of tropomyosin residue 146 to residues 157-163 in the C-terminal domain of troponin I in reconstituted skeletal muscle thin filaments
Abstract
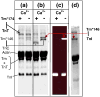
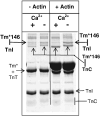
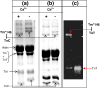
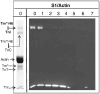
The Ca(2+)-dependent interaction of troponin I (TnI) with actin.tropomyosin (Tm) in muscle thin filaments is a critical step in the regulation of muscle contraction. Previous studies have suggested that, in the absence of Ca(2+), TnI interacts with Tm and actin in reconstituted muscle thin filaments, maintaining Tm at the outer domain of actin and blocking myosin-actin interaction. To obtain direct evidence for this Tm-TnI interaction, we performed photochemical crosslinking studies using Tm labeled with 4-maleimidobenzophenone at position 146 or 174 (Tm*146 or Tm*174, respectively), reconstituted with actin and troponin [composed of TnI, troponin T (TnT), and troponin C] or with actin and TnI. After near-UV irradiation, SDS gels of the Tm*146-containing thin filament showed three new high-molecular-weight bands determined to be crosslinked products Tm*146-TnI, Tm*146-troponin C, and Tm*146-TnT using fluorescence-labeled TnI, mass spectrometry, and Western blot analysis. While Tm*146-TnI was produced only in the absence of Ca(2+), the production of other crosslinked species did not show Ca(2+) dependence. Tm*174 mainly crosslinked to TnT. In the absence of actin, a similar crosslinking pattern was obtained with a much lower yield. A tryptic peptide from Tm*146-TnI with a molecular mass of 2601.2 Da that was not present in the tryptic peptides of Tm*146 or TnI was identified using HPLC and matrix-assisted laser desorption/ionization time-of-flight. This was shown, using absorption and fluorescence spectroscopy, to be the 4-maleimidobenzophenone-labeled peptide from Tm crosslinked to TnI peptide 157-163. These data, which show that a region in the C-terminal domain of TnI interacts with Tm in the absence of Ca(2+), support the hypothesis that a TnI-Tm interaction maintains Tm at the outer domain of actin and will help efforts to localize troponin in actin.Tm muscle thin filaments.
Figures


References
-
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. - PubMed
-
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. - PubMed
-
- Luo Y, Leszyk J, Li B, Li Z, Gergely J, Tao T. Troponin-I interacts with the Met47 region of skeletal muscle actin. Implications for the mechanism of thin filament regulation by calcium. J Mol Biol. 2002;316:429–34. - PubMed
-
- Szczesna D, Potter JD. The role of troponin in the Ca(2+)-regulation of skeletal muscle contraction. Results Probl Cell Differ. 2002;36:171–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

