Decision by division: making cortical maps
- PMID: 19380167
- PMCID: PMC3601545
- DOI: 10.1016/j.tins.2009.01.007
Decision by division: making cortical maps
Abstract
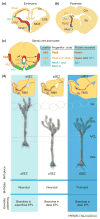
In the past three decades, mounting evidence has revealed that specification of the basic cortical neuronal classes starts at the time of their final mitotic divisions in the embryonic proliferative zones. This early cell determination continues during the migration of the newborn neurons across the widening cerebral wall, and it is in the cortical plate that they attain their final positions and establish species-specific cytoarchitectonic areas. Here, the development and evolutionary expansion of the neocortex is viewed in the context of the radial unit and protomap hypotheses. A broad spectrum of findings gave insight into the pathogenesis of cortical malformations and the biological bases for the evolution of the modern human neocortex. We examine the history and evidence behind the concept of early specification of neurons and provide the latest compendium of genes and signaling molecules involved in neuronal fate determination and specification.
Figures




References
-
- Kuan CY, et al. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. - PubMed
-
- Rakic P. Neuronal–glial interaction during brain development. Trends Neurosci. 1981;4:184–187.
-
- Rakic P. Mechanism of ocular dominance segregation in the lateral geniculate nucleus: competitive elimination hypothesis. Trends Neurosci. 1986;9:11–15.
-
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. - PubMed
-
- Sarkisian MR, et al. Trouble making the first move: interpreting arrested neuronal migration in the cerebral cortex. Trends Neurosci. 2008;31:54–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

