Light-induced vegetative anthocyanin pigmentation in Petunia
- PMID: 19380423
- PMCID: PMC2682507
- DOI: 10.1093/jxb/erp097
Light-induced vegetative anthocyanin pigmentation in Petunia
Abstract
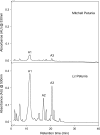
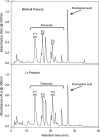
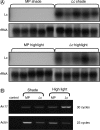
The Lc petunia system, which displays enhanced, light-induced vegetative pigmentation, was used to investigate how high light affects anthocyanin biosynthesis, and to assess the effects of anthocyanin pigmentation upon photosynthesis. Lc petunia plants displayed intense purple anthocyanin pigmentation throughout the leaves and stems when grown under high-light conditions, yet remain acyanic when grown under shade conditions. The coloured phenotypes matched with an accumulation of anthocyanins and flavonols, as well as the activation of the early and late flavonoid biosynthetic genes required for flavonol and anthocyanin production. Pigmentation in Lc petunia only occurred under conditions which normally induce a modest amount of anthocyanin to accumulate in wild-type Mitchell petunia [Petunia axillaris x (Petunia axillaris x Petunia hybrida cv. 'Rose of Heaven')]. Anthocyanin pigmentation in Lc petunia leaves appears to screen underlying photosynthetic tissues, increasing light saturation and light compensation points, without reducing the maximal photosynthetic assimilation rate (A(max)). In the Lc petunia system, where the bHLH factor Leaf colour is constitutively expressed, expression of the bHLH (Lc) and WD40 (An11) components of the anthocyanin regulatory system were not limited, suggesting that the high-light-induced anthocyanin pigmentation is regulated by endogenous MYB transcription factors.
Figures




References
-
- Aida R, Yoshida K, Kondo T, Kishimoto S, Shibata M. Copigmentation gives bluer flowers on transgenic torenia plants with the antisense dihydroflavonol-4-reductase gene. Plant Science. 2000;160:49–56. - PubMed
-
- Ausubel FM, Bahnsen K, Hanson M, Mitchell A, Smith HJ. Cell and tissue culture of haploid and diploid Petunia ‘Mitchell’. Plant Molecular Biology Newsletter. 1980;1:26–32.
-
- Bloor SJ, Bradley JM, Lewis DH, Davies KM. Identification of flavonol and anthocyanin metabolites in leaves of Petunia ‘Mitchell’ and its Lc transgenic. Phytochemistry. 1998;49:1427–1430.
-
- Boase MR, Bradley JM, Borst NK. Genetic transformation mediated by Agrobacterium tumefaciens of florists’ chrysanthemum (Dendranthema grandiflorum) cultivar ‘Peach Margaret’. In Vitro Cellular and Developmental Biology-Plant. 1998;34:46–51.

