CXCR3 activation by lentivirus infection suppresses neuronal autophagy: neuroprotective effects of antiretroviral therapy
- PMID: 19380511
- PMCID: PMC2796902
- DOI: 10.1096/fj.08-128819
CXCR3 activation by lentivirus infection suppresses neuronal autophagy: neuroprotective effects of antiretroviral therapy
Abstract
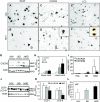
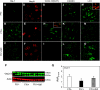
Previous studies have implicated CXCL12 in the neuropathogenesis of HIV infection. Proteolysis of CXCL12 generates a neurotoxic molecule, CXCL12(5-67), which engages and activates CXCR3, in addition to exhibiting increased expression in the brains of patients with HIV-associated dementia (HAD). Herein, we investigated CXCR3-mediated neuronal injury, particularly, its contribution to autophagy suppression and the concomitant effects of antiretroviral therapy using human brain samples and models of HIV neuropathogenesis. Neurons in the brains of HAD patients and feline immunodeficiency virus (FIV)-infected animals, as well as cultured human neurons, expressed CXCR3, which was modulated in a ligand-specific manner. Exposure of human neurons to CXCL12(5-67) caused a reduction in the autophagy-associated molecule LC3 (P<0.05) and neuronal survival (P<0.05), which recapitulated findings in FIV- and HIV-infected brains (P<0.05). Oral didanosine (ddI) treatment of FIV-infected animals reduced neurobehavioral abnormalities in conjunction with diminished plasma viral load (P<0.05). F4/80 transcript abundance and CXCL12(5-67) immunoreactivity were reduced with restored neuronal LC3 expression in the brains of FIV-infected animals after ddI treatment (P<0.05). ddI treatment also prevented microglial activation and depletion of synaptic proteins in the cortex of FIV-infected animals (P<0.05). These findings indicate that the beneficial effects of ddI might be a consequence of a reduced systemic viral burden and concurrent leukocyte activation, leading to diminished neuroinflammation with preservation of neuronal autophagy by regulating CXCR3 activation.
Figures


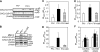
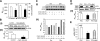

Similar articles
-
Neurologic disease in feline immunodeficiency virus infection: disease mechanisms and therapeutic interventions for NeuroAIDS.J Neurovirol. 2018 Apr;24(2):220-228. doi: 10.1007/s13365-017-0593-1. Epub 2017 Dec 15. J Neurovirol. 2018. PMID: 29247305 Review.
-
Regulation of lentivirus neurovirulence by lipopolysaccharide conditioning: suppression of CXCL10 in the brain by IL-10.J Immunol. 2010 Feb 1;184(3):1566-74. doi: 10.4049/jimmunol.0902575. Epub 2009 Dec 30. J Immunol. 2010. PMID: 20042580
-
Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression.Brain. 2007 Aug;130(Pt 8):2011-23. doi: 10.1093/brain/awm148. Epub 2007 Jul 6. Brain. 2007. PMID: 17616550
-
Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration.J Neurosci. 2016 Oct 12;36(41):10683-10695. doi: 10.1523/JNEUROSCI.1287-16.2016. J Neurosci. 2016. PMID: 27733618 Free PMC article.
-
Comparative neurovirulence in lentiviral infections: The roles of viral molecular diversity and select proteases.J Neurovirol. 2004;10 Suppl 1:113-7. doi: 10.1080/753312762. J Neurovirol. 2004. PMID: 14982749 Review.
Cited by
-
CXCR3 Inhibition Blocks the NF-κB Signaling Pathway by Elevating Autophagy to Ameliorate Lipopolysaccharide-Induced Intestinal Dysfunction in Mice.Cells. 2023 Jan 1;12(1):182. doi: 10.3390/cells12010182. Cells. 2023. PMID: 36611975 Free PMC article.
-
Differential type 1 interferon-regulated gene expression in the brain during AIDS: interactions with viral diversity and neurovirulence.FASEB J. 2013 Jul;27(7):2829-44. doi: 10.1096/fj.13-227868. Epub 2013 Apr 22. FASEB J. 2013. PMID: 23608145 Free PMC article.
-
Neurologic disease in feline immunodeficiency virus infection: disease mechanisms and therapeutic interventions for NeuroAIDS.J Neurovirol. 2018 Apr;24(2):220-228. doi: 10.1007/s13365-017-0593-1. Epub 2017 Dec 15. J Neurovirol. 2018. PMID: 29247305 Review.
-
Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator.J Neuroinflammation. 2012 Oct 18;9:239. doi: 10.1186/1742-2094-9-239. J Neuroinflammation. 2012. PMID: 23078780 Free PMC article.
-
Dual role of autophagy in HIV-1 replication and pathogenesis.AIDS Res Ther. 2012 May 20;9(1):16. doi: 10.1186/1742-6405-9-16. AIDS Res Ther. 2012. PMID: 22606989 Free PMC article.
References
-
- Rock R B, Peterson P K. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J Neuroimmune Pharmacol. 2006;1:117–126. - PubMed
-
- Fehder W P, Douglas S D. Interactions between the nervous and immune systems. Semin Clin Neuropsychiatry. 2001;6:229–240. - PubMed
-
- Noorbakhsh F, Overall C M, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. - PubMed
-
- Ryan L A, Cotter R L, Zink W E, 2nd, Gendelman H E, Zheng J. Macrophages, chemokines and neuronal injury in HIV-1-associated dementia. Cell Mol Biol (Noisy-le-grand) 2002;48:137–150. - PubMed
-
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20:3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

