Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin
- PMID: 19380584
- PMCID: PMC2708885
- DOI: 10.1074/jbc.M109.009589
Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin
Abstract
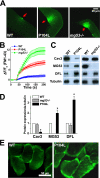
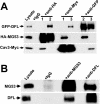
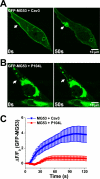
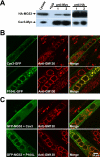
Defective membrane repair can contribute to the progression of muscular dystrophy. Although mutations in caveolin-3 (Cav3) and dysferlin are linked to muscular dystrophy in human patients, the molecular mechanism underlying the functional interplay between Cav3 and dysferlin in membrane repair of muscle physiology and disease has not been fully resolved. We recently discovered that mitsugumin 53 (MG53), a muscle-specific TRIM (Tri-partite motif) family protein (TRIM72), contributes to intracellular vesicle trafficking and is an essential component of the membrane repair machinery in striated muscle. Here we show that MG53 interacts with dysferlin and Cav3 to regulate membrane repair in skeletal muscle. MG53 mediates active trafficking of intracellular vesicles to the sarcolemma and is required for movement of dysferlin to sites of cell injury during repair patch formation. Mutations in Cav3 (P104L, R26Q) that cause retention of Cav3 in Golgi apparatus result in aberrant localization of MG53 and dysferlin in a dominant-negative fashion, leading to defective membrane repair. Our data reveal that a molecular complex formed by MG53, dysferlin, and Cav3 is essential for repair of muscle membrane damage and also provide a therapeutic target for treatment of muscular and cardiovascular diseases that are linked to compromised membrane repair.
Figures


References
-
- McNeil P. L., Kirchhausen T. ( 2005) Nat. Rev. Mol. Cell Biol. 6, 499– 505 - PubMed
-
- Towler M. C., Kaufman S. J., Brodsky F. M. ( 2004) Traffic 5, 129– 139 - PubMed
-
- Glover L., Brown R. H., Jr.( 2007) Traffic 8, 785– 794 - PubMed
-
- Steinhardt R. A., Bi G., Alderton J. M. ( 1994) Science 263, 390– 393 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

