T follicular helper cells differentiate from Th2 cells in response to helminth antigens
- PMID: 19380637
- PMCID: PMC2715032
- DOI: 10.1084/jem.20090303
T follicular helper cells differentiate from Th2 cells in response to helminth antigens
Abstract
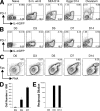
The relationship of T follicular helper (TFH) cells to other T helper (Th) subsets is controversial. We find that after helminth infection, or immunization with helminth antigens, reactive lymphoid organs of 4get IL-4/GFP reporter mice contain populations of IL-4/GFP-expressing CD4(+) T cells that display the TFH markers CXCR5, PD-1, and ICOS. These TFH cells express the canonical TFH markers BCL6 and IL-21, but also GATA3, the master regulator of Th2 cell differentiation. Consistent with a relationship between Th2 and TFH cells, IL-4 protein production, reported by expression of huCD2 in IL-4 dual reporter (4get/KN2) mice, was a robust marker of TFH cells in LNs responding to helminth antigens. Moreover, the majority of huCD2/IL-4-producing Th cells were found within B cell follicles, consistent with their definition as TFH cells. TFH cell development after immunization failed to occur in mice lacking B cells or CD154. The relationship of TFH cells to the Th2 lineage was confirmed when TFH cells were found to develop from CXCR5(-) PD-1(-) IL-4/GFP(+) CD4(+) T cells after their transfer into naive mice and antigen challenge in vivo.
Figures




References
-
- Finkelman F.D., Holmes J., Katona I.M., Urban J.F., Jr., Beckmann M.P., Park L.S., Schooley K.A., Coffman R.L., Mosmann T.R., Paul W.E. 1990. Lymphokine control of in vivo immunoglobulin isotype selection.Annu. Rev. Immunol. 8:303–333 - PubMed
-
- King C., Tangye S.G., Mackay C.R. 2008. T follicular helper (TFH) cells in normal and dysregulated immune responses.Annu. Rev. Immunol. 26:741–766 - PubMed
-
- Chtanova T., Tangye S.G., Newton R., Frank N., Hodge M.R., Rolph M.S., Mackay C.R. 2004. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells.J. Immunol. 173:68–78 - PubMed
-
- Kim C.H., Lim H.W., Kim J.R., Rott L., Hillsamer P., Butcher E.C. 2004. Unique gene expression program of human germinal center T helper cells.Blood. 104:1952–1960 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

