TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma
- PMID: 19380639
- PMCID: PMC2715030
- DOI: 10.1084/jem.20090528
TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma
Abstract
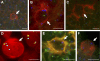
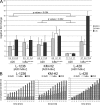
Proliferation and survival of Hodgkin and Reed/Sternberg (HRS) cells, the malignant cells of classical Hodgkin lymphoma (cHL), are dependent on constitutive activation of nuclear factor kappaB (NF-kappaB). NF-kappaB activation through various stimuli is negatively regulated by the zinc finger protein A20. To determine whether A20 contributes to the pathogenesis of cHL, we sequenced TNFAIP3, encoding A20, in HL cell lines and laser-microdissected HRS cells from cHL biopsies. We detected somatic mutations in 16 out of 36 cHLs (44%), including missense mutations in 2 out of 16 Epstein-Barr virus-positive (EBV(+)) cHLs and a missense mutation, nonsense mutations, and frameshift-causing insertions or deletions in 14 out of 20 EBV(-) cHLs. In most mutated cases, both TNFAIP3 alleles were inactivated, including frequent chromosomal deletions of TNFAIP3. Reconstitution of wild-type TNFAIP3 in A20-deficient cHL cell lines revealed a significant decrease in transcripts of selected NF-kappaB target genes and caused cytotoxicity. Extending the mutation analysis to primary mediastinal B cell lymphoma (PMBL), another lymphoma with constitutive NF-kappaB activity, revealed destructive mutations in 5 out of 14 PMBLs (36%). This report identifies TNFAIP3 (A20), a key regulator of NF-kappaB activity, as a novel tumor suppressor gene in cHL and PMBL. The significantly higher frequency of TNFAIP3 mutations in EBV(-) than EBV(+) cHL suggests complementing functions of TNFAIP3 inactivation and EBV infection in cHL pathogenesis.
Figures

References
-
- Bargou R.C., Emmerich F., Krappmann D., Bommert K., Mapara M.Y., Arnold W., Royer H.D., Grinstein E., Greiner A., Scheidereit C., Dorken B. 1997. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells.J. Clin. Invest. 100:2961–2969 - PMC - PubMed
-
- Hinz M., Lemke P., Anagnostopoulos I., Hacker C., Krappmann D., Mathas S., Dorken B., Zenke M., Stein H., Scheidereit C. 2002. Nuclear factor κB–dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity.J. Exp. Med. 196:605–617 - PMC - PubMed
-
- Hayden M.S., Ghosh S. 2008. Shared principles in NF-kappaB signaling.Cell. 132:344–362 - PubMed
-
- Wu C.J., Conze D.B., Li T., Srinivasula S.M., Ashwell J.D. 2006. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation.Nat. Cell Biol. 8:398–406 - PubMed
-
- Ea C.K., Deng L., Xia Z.P., Pineda G., Chen Z.J. 2006. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO.Mol. Cell. 22:245–257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

