Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity
- PMID: 19380744
- PMCID: PMC2670884
- DOI: 10.1073/pnas.0902749106
Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity
Abstract
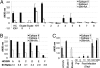
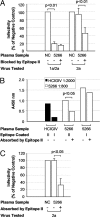
Using human immune globulins made from antihepatitis C virus (HCV)-positive plasma, we recently identified two antibody epitopes in the E2 protein at residues 412-426 (epitope I) and 434-446 (epitope II). Whereas epitope I is highly conserved among genotypes, epitope II varies. We discovered that epitope I was implicated in HCV neutralization whereas the binding of non-neutralizing antibody to epitope II disrupted virus neutralization mediated by antibody binding at epitope I. These findings suggested that, if this interfering mechanism operates in vivo during HCV infection, a neutralizing antibody against epitope I can be restrained by an interfering antibody, which may account for the persistence of HCV even in the presence of an abundance of neutralizing antibodies. We tested this hypothesis by affinity depletion and peptide-blocking of epitope-II-specific antibodies in plasma of a chronically HCV-infected patient and recombinant E1E2 vaccinated chimpanzees. We demonstrate that, by removing the restraints imposed by the interfering antibodies to epitope-II, neutralizing activity can be revealed in plasma that previously failed to neutralize viral stock in cell culture. Further, cross-genotype neutralization could be generated from monospecific plasma. Our studies contribute to understanding the mechanisms of antibody-mediated neutralization and interference and provide a practical approach to the development of more potent and broadly reactive hepatitis C immune globulins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
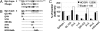

References
-
- Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35. - PubMed
-
- Davis GL. Hepatitis C immune globulin to prevent HCV recurrence after liver transplantation: chasing windmills? Liver Transpl. 2006;12:1317–1319. - PubMed
-
- Triyatni M, et al. Structural features of envelope proteins on hepatitis C virus-like particles as determined by anti-envelope monoclonal antibodies and CD81 binding. Virology. 2002;298:124–132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

