Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay
- PMID: 19380751
- PMCID: PMC2672556
- DOI: 10.1073/pnas.0810878106
Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay
Abstract
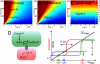
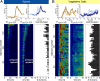
A common form of quorum sensing in gram-positive bacteria is mediated by peptides that act as phosphatase regulators (Phr) of receptor aspartyl phosphatases (Raps). In Bacillus subtilis, several Phr signals are integrated in sporulation phosphorelay signal transduction. We theoretically demonstrate that the phosphorelay can act as a computational machine performing a sensitive division operation of kinase-encoded signals by quorum-modulated Rap signals, indicative of cells computing a "food per cell" estimate to decide whether to enter sporulation. We predict expression from the rapA-phrA operon to bifurcate as relative environmental signals change in a developing population. We experimentally observe that the rapA-phrA operon is heterogeneously induced in sporulating microcolonies. Uninduced cells sporulate rather synchronously early on, whereas the RapA/PhrA subpopulation sporulates less synchronously throughout later stationary phase. Moreover, we show that cells sustain PhrA expression during periods of active growth. Together with the model, these findings suggest that the phosphorelay may normalize environmental signals by the size of the (sub)population actively competing for nutrients (as signaled by PhrA). Generalizing this concept, the various Phrs could facilitate subpopulation communication in dense isogenic communities to control the physiological strategies followed by differentiated subpopulations by interpreting (environmental) signals based on the spatiotemporal community structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
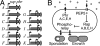


References
-
- Pottathil M, Lazazzera BA. The extracellular Phr peptide-rap phosphatase signaling circuit of Bacillus subtilis. Front Biosci. 2003;8:D32–D45. - PubMed
-
- Perego M, Higgins CF, Pearce SR, Gallagher MP, Hoch JA. The oligopeptide transport-system of Bacillus-subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991;5(1):173–185. - PubMed
-
- Lazazzera BA, Solomon JM, Grossman AD. An exported peptide functions intracellularly to contribute to cell density signaling in B-subtilis. Cell. 1997;89(6):917–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

