PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients
- PMID: 19380770
- PMCID: PMC3426222
- DOI: 10.4049/jimmunol.0803245
PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients
Abstract
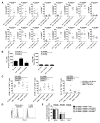
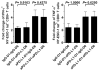
The programmed death 1 (PD-1) receptor is a negative regulator of activated T cells and is up-regulated on exhausted virus-specific CD8(+) T cells in chronically infected mice and humans. Programmed death ligand 1 (PD-L1) is expressed by multiple tumors, and its interaction with PD-1 resulted in tumor escape in experimental models. To investigate the role of PD-1 in impairing spontaneous tumor Ag-specific CD8(+) T cells in melanoma patients, we have examined the effect of PD-1 expression on ex vivo detectable CD8(+) T cells specific to the tumor Ag NY-ESO-1. In contrast to EBV, influenza, or Melan-A/MART-1-specific CD8(+) T cells, NY-ESO-1-specific CD8(+) T cells up-regulated PD-1 expression. PD-1 up-regulation on spontaneous NY-ESO-1-specific CD8(+) T cells occurs along with T cell activation and is not directly associated with an inability to produce cytokines. Importantly, blockade of the PD-1/PD-L1 pathway in combination with prolonged Ag stimulation with PD-L1(+) APCs or melanoma cells augmented the number of cytokine-producing, proliferating, and total NY-ESO-1-specific CD8(+) T cells. Collectively, our findings support the role of PD-1 as a regulator of NY-ESO-1-specific CD8(+) T cell expansion in the context of chronic Ag stimulation. They further support the use of PD-1/PD-L1 pathway blockade in cancer patients to partially restore NY-ESO-1-specific CD8(+) T cell numbers and functions, increasing the likelihood of tumor regression.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. - PubMed
-
- Haas GG, Jr, D’Cruz OJ, De Bault LE. Distribution of human leukocyte antigen-ABC and -D/DR antigens in the unfixed human testis. Am J Reprod Immunol Microbiol. 1988;18:47–51. - PubMed
-
- Jager E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jager D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

