LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen
- PMID: 19380791
- PMCID: PMC2703443
- DOI: 10.4049/jimmunol.0801165
LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen
Abstract
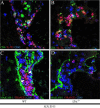
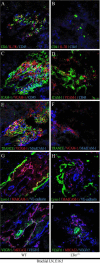
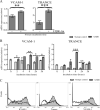
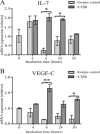
The formation of lymph nodes is a complex process crucially controlled through triggering of LTbetaR on mesenchymal cells by LTalpha(1)beta(2) expressing lymphoid tissue inducer (LTi) cells. This leads to the induction of chemokines to attract more hematopoietic cells and adhesion molecules to retain them. In this study, we show that the extravasation of the first hematopoietic cells at future lymph node locations occurs independently of LTalpha and that these cells, expressing TNF-related activation-induced cytokine (TRANCE), are the earliest LTi cells. By paracrine signaling the first expression of LTalpha(1)beta(2) is induced. Subsequent LTbetaR triggering on mesenchymal cells leads to their differentiation to stromal organizers, which now also start to express TRANCE, IL-7, as well as VEGF-C, in addition to the induced adhesion molecules and chemokines. Both TRANCE and IL-7 will further induce the expression of LTalpha(1)beta(2) on newly arrived immature LTi cells, resulting in more LTbetaR triggering, generating a positive feedback loop. Thus, LTbetaR triggering by LTi cells during lymph node development creates a local environment to which hematopoietic precursors are attracted and where they locally differentiate into fully mature, LTalpha(1)beta(2) expressing, LTi cells. Furthermore, the same signals may regulate lymphangiogenesis to the lymph node through induction of VEGF-C.
Figures

References
-
- Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer's patch formation of murine embryo. Int. Immunol. 1997;9:507–514. - PubMed
-
- Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S, Nishikawa S. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–833. - PubMed
-
- Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor α in the formation of Peyer's patch anlage. Int. Immunol. 1998;10:1–6. - PubMed
-
- Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor α+ CD3 − cells in the embryonic intestine induces the organizing center of Peyer's patches. t. Immunol. 1999;11:643–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

