Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members
- PMID: 19380879
- PMCID: PMC2700382
- DOI: 10.1083/jcb.200809153
Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members
Abstract
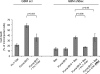
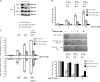
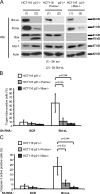
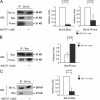
It is still unclear whether the BH3-only protein Puma (p53 up-regulated modulator of apoptosis) can prime cells to death and render antiapoptotic BH3-binding Bcl-2 homologues necessary for survival through its ability to directly interact with proapoptotic Bax and activate it. In this study, we provide further evidence, using cell-free assays, that the BH3 domain of Puma binds Bax at an activation site that comprises the first helix of Bax. We also show that, in yeast, Puma interacts with Bax and triggers its killing activity when Bcl-2 homologues are absent but not when Bcl-xL is expressed. Finally, endogenous Puma is involved in the apoptotic response of human colorectal cancer cells to the Bcl-2/Bcl-xL inhibitor ABT-737, even in conditions where the expression of Mcl-1 is down-regulated. Thus, Puma is competent to trigger Bax activity by itself, thereby promoting cellular dependence on prosurvival Bcl-2 family members.
Figures



Comment in
-
Puma strikes Bax.J Cell Biol. 2009 Apr 20;185(2):189-91. doi: 10.1083/jcb.200903134. J Cell Biol. 2009. PMID: 19380876 Free PMC article.
References
-
- Arokium H., Camougrand N., Vallette F.M., Manon S. 2004. Studies of the interaction of substituted mutants of BAX with yeast mitochondria reveal that the C-terminal hydrophobic alpha-helix is a second ART sequence and plays a role in the interaction with anti-apoptotic BCL-xL.J. Biol. Chem. 279:52566–52573 - PubMed
-
- Arokium H., Ouerfelli H., Velours G., Camougrand N., Vallette F.M., Manon S. 2007. Substitutions of potentially phosphorylatable serine residues of Bax reveal how they may regulate its interaction with mitochondria.J. Biol. Chem. 282:35104–35112 - PubMed
-
- Bellot G., Cartron P.F., Er E., Oliver L., Juin P., Armstrong L.C., Bornstein P., Mihara K., Manon S., Vallette F.M. 2007. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax.Cell Death Differ. 14:785–794 - PubMed
-
- Brix J., Rudiger S., Bukau B., Schneider-Mergener J., Pfanner N. 1999. Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein.J. Biol. Chem. 274:16522–16530 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

