Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39
- PMID: 19381014
- PMCID: PMC2673847
- DOI: 10.1172/JCI36433
Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39
Abstract
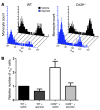
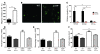
Leukocyte and platelet accumulation at sites of cerebral ischemia exacerbate cerebral damage. The ectoenzyme CD39 on the plasmalemma of endothelial cells metabolizes ADP to suppress platelet accumulation in the ischemic brain. However, the role of leukocyte surface CD39 in regulating monocyte and neutrophil trafficking in this setting is not known. Here we have demonstrated in mice what we believe to be a novel mechanism by which CD39 on monocytes and neutrophils regulates their own sequestration into ischemic cerebral tissue, by catabolizing nucleotides released by injured cells, thereby inhibiting their chemotaxis, adhesion, and transmigration. Bone marrow reconstitution and provision of an apyrase, an enzyme that hydrolyzes nucleoside tri- and diphosphates, each normalized ischemic leukosequestration and cerebral infarction in CD39-deficient mice. Leukocytes purified from Cd39-/- mice had a markedly diminished capacity to phosphohydrolyze adenine nucleotides and regulate platelet reactivity, suggesting that leukocyte ectoapyrases modulate the ambient vascular nucleotide milieu. Dissipation of ATP by CD39 reduced P2X7 receptor stimulation and thereby suppressed baseline leukocyte alphaMbeta2-integrin expression. As alphaMbeta2-integrin blockade reversed the postischemic, inflammatory phenotype of Cd39-/- mice, these data suggest that phosphohydrolytic activity on the leukocyte surface suppresses cell-cell interactions that would otherwise promote thrombosis or inflammation. These studies indicate that CD39 on both endothelial cells and leukocytes reduces inflammatory cell trafficking and platelet reactivity, with a consequent reduction in tissue injury following cerebral ischemic challenge.
Figures







References
-
- Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL046403/HL/NHLBI NIH HHS/United States
- T32 HL007853/HL/NHLBI NIH HHS/United States
- HL46403/HL/NHLBI NIH HHS/United States
- R01 NS041460/NS/NINDS NIH HHS/United States
- P01HL089407/HL/NHLBI NIH HHS/United States
- HL47073/HL/NHLBI NIH HHS/United States
- R37 HL047073/HL/NHLBI NIH HHS/United States
- HL086676/HL/NHLBI NIH HHS/United States
- R01 HL086676/HL/NHLBI NIH HHS/United States
- R01 HL069448/HL/NHLBI NIH HHS/United States
- NS041460/NS/NINDS NIH HHS/United States
- R01 HL047073/HL/NHLBI NIH HHS/United States
- HL69448/HL/NHLBI NIH HHS/United States
- P01 HL089407/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

