Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process
- PMID: 19381016
- PMCID: PMC2673871
- DOI: 10.1172/JCI37284
Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process
Erratum in
- J Clin Invest. 2010 Mar 1;120(3):931-2
Abstract
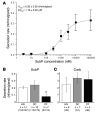
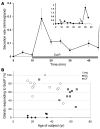
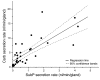
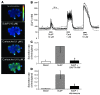
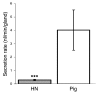
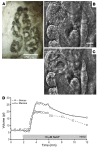
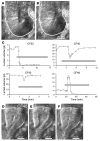
Chronic bacterial airway infections are the major cause of mortality in cystic fibrosis (CF). Normal airway defenses include reflex stimulation of submucosal gland mucus secretion by sensory neurons that release substance P (SubP). CFTR is an anion channel involved in fluid secretion and mutated in CF; the role of CFTR in secretions stimulated by SubP is unknown. We used optical methods to measure SubP-mediated secretion from human submucosal glands in lung transplant tissue. Glands from control but not CF subjects responded to mucosal chili oil. Similarly, serosal SubP stimulated secretion in more than 60% of control glands but only 4% of CF glands. Secretion triggered by SubP was synergistic with vasoactive intestinal peptide and/or forskolin but not with carbachol; synergy was absent in CF glands. Pig glands demonstrated a nearly 10-fold greater response to SubP. In 10 of 11 control glands isolated by fine dissection, SubP caused cell volume loss, lumen expansion, and mucus flow, but in 3 of 4 CF glands, it induced lumen narrowing. Thus, in CF, the reduced ability of mucosal irritants to stimulate airway gland secretion via SubP may be another factor that predisposes the airways to infections.
Figures



Similar articles
-
Properties of substance P-stimulated mucus secretion from porcine tracheal submucosal glands.Am J Physiol Lung Cell Mol Physiol. 2011 Mar;300(3):L370-9. doi: 10.1152/ajplung.00372.2010. Epub 2010 Dec 3. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21131402
-
Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator knockout mice.J Physiol. 2007 Apr 1;580(Pt 1):301-14. doi: 10.1113/jphysiol.2006.123653. Epub 2007 Jan 4. J Physiol. 2007. PMID: 17204498 Free PMC article.
-
Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis.J Clin Invest. 2007 Oct;117(10):3118-27. doi: 10.1172/JCI31992. J Clin Invest. 2007. PMID: 17853942 Free PMC article.
-
HCO3- transport in relation to mucus secretion from submucosal glands.JOP. 2001 Jul;2(4 Suppl):280-4. JOP. 2001. PMID: 11875272 Review.
-
Fluid secretion by submucosal glands of the tracheobronchial airways.Respir Physiol Neurobiol. 2007 Dec 15;159(3):271-7. doi: 10.1016/j.resp.2007.06.017. Epub 2007 Jul 7. Respir Physiol Neurobiol. 2007. PMID: 17707699 Free PMC article. Review.
Cited by
-
Nebulized hypertonic saline triggers nervous system-mediated active liquid secretion in cystic fibrosis swine trachea.Sci Rep. 2019 Jan 24;9(1):540. doi: 10.1038/s41598-018-36695-4. Sci Rep. 2019. PMID: 30679487 Free PMC article.
-
CFTR, mucins, and mucus obstruction in cystic fibrosis.Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a009589. doi: 10.1101/cshperspect.a009589. Cold Spring Harb Perspect Med. 2012. PMID: 22951447 Free PMC article. Review.
-
Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis.Respir Res. 2018 Aug 6;19(1):149. doi: 10.1186/s12931-018-0846-4. Respir Res. 2018. PMID: 30081920 Free PMC article. Review.
-
Why mouse airway submucosal gland serous cells do not secrete fluid in response to cAMP stimulation.J Biol Chem. 2012 Nov 2;287(45):38316-26. doi: 10.1074/jbc.M112.412817. Epub 2012 Sep 18. J Biol Chem. 2012. PMID: 22989883 Free PMC article.
-
Effects of secretagogues on net and unidirectional liquid fluxes across porcine bronchial airways.Am J Physiol Lung Cell Mol Physiol. 2010 Feb;298(2):L270-6. doi: 10.1152/ajplung.00253.2009. Epub 2009 Nov 13. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 19915159 Free PMC article.

