Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice
- PMID: 19381018
- PMCID: PMC2673879
- DOI: 10.1172/JCI38022
Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice
Erratum in
- J Clin Invest. 2012 Apr 2;122(4):1584. Heller Brown, Joan [corrected to Brown, Joan Heller]
Abstract
Ca2+/calmodulin-dependent kinase II (CaMKII) has been implicated in cardiac hypertrophy and heart failure. We generated mice in which the predominant cardiac isoform, CaMKIIdelta, was genetically deleted (KO mice), and found that these mice showed no gross baseline changes in ventricular structure or function. In WT and KO mice, transverse aortic constriction (TAC) induced comparable increases in relative heart weight, cell size, HDAC5 phosphorylation, and hypertrophic gene expression. Strikingly, while KO mice showed preserved hypertrophy after 6-week TAC, CaMKIIdelta deficiency significantly ameliorated phenotypic changes associated with the transition to heart failure, such as chamber dilation, ventricular dysfunction, lung edema, cardiac fibrosis, and apoptosis. The ratio of IP3R2 to ryanodine receptor 2 (RyR2) and the fraction of RyR2 phosphorylated at the CaMKII site increased significantly during development of heart failure in WT mice, but not KO mice, and this was associated with enhanced Ca2+ spark frequency only in WT mice. We suggest that CaMKIIdelta contributes to cardiac decompensation by enhancing RyR2-mediated sarcoplasmic reticulum Ca2+ leak and that attenuating CaMKIIdelta activation can limit the progression to heart failure.
Figures
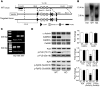



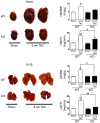

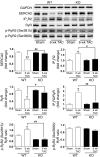

Comment in
-
CaMKII and a failing strategy for growth in heart.J Clin Invest. 2009 May;119(5):1082-5. doi: 10.1172/jci39262. J Clin Invest. 2009. PMID: 19422097 Free PMC article.
Similar articles
-
CaMKIIδ mediates β-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic β-adrenergic stimulation.J Mol Cell Cardiol. 2015 Aug;85:282-91. doi: 10.1016/j.yjmcc.2015.06.007. Epub 2015 Jun 14. J Mol Cell Cardiol. 2015. PMID: 26080362 Free PMC article.
-
CaMKIIδC Drives Early Adaptive Ca2+ Change and Late Eccentric Cardiac Hypertrophy.Circ Res. 2020 Oct 9;127(9):1159-1178. doi: 10.1161/CIRCRESAHA.120.316947. Epub 2020 Aug 21. Circ Res. 2020. PMID: 32821022 Free PMC article.
-
Ca2+/calmodulin-dependent kinase IIδC-induced chronic heart failure does not depend on sarcoplasmic reticulum Ca2+ leak.ESC Heart Fail. 2024 Aug;11(4):2191-2199. doi: 10.1002/ehf2.14772. Epub 2024 Apr 14. ESC Heart Fail. 2024. PMID: 38616546 Free PMC article.
-
Cardiac hypertrophy and heart failure development through Gq and CaM kinase II signaling.J Cardiovasc Pharmacol. 2010 Dec;56(6):598-603. doi: 10.1097/FJC.0b013e3181e1d263. J Cardiovasc Pharmacol. 2010. PMID: 20531218 Free PMC article. Review.
-
Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes?Recent Prog Horm Res. 2004;59:141-68. doi: 10.1210/rp.59.1.141. Recent Prog Horm Res. 2004. PMID: 14749501 Review.
Cited by
-
New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII).Circulation. 2012 Oct 23;126(17):2125-39. doi: 10.1161/CIRCULATIONAHA.112.124990. Circulation. 2012. PMID: 23091085 Free PMC article. Review. No abstract available.
-
Loss of myocardial retinoic acid receptor α induces diastolic dysfunction by promoting intracellular oxidative stress and calcium mishandling in adult mice.J Mol Cell Cardiol. 2016 Oct;99:100-112. doi: 10.1016/j.yjmcc.2016.08.009. Epub 2016 Aug 15. J Mol Cell Cardiol. 2016. PMID: 27539860 Free PMC article.
-
CaMKIIδ mediates β-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic β-adrenergic stimulation.J Mol Cell Cardiol. 2015 Aug;85:282-91. doi: 10.1016/j.yjmcc.2015.06.007. Epub 2015 Jun 14. J Mol Cell Cardiol. 2015. PMID: 26080362 Free PMC article.
-
Ca2+/Calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB.Circ Res. 2013 Mar 15;112(6):935-44. doi: 10.1161/CIRCRESAHA.112.276915. Epub 2013 Feb 6. Circ Res. 2013. PMID: 23388157 Free PMC article.
-
CaMKII-δ9 Induces Cardiomyocyte Death to Promote Cardiomyopathy and Heart Failure.Front Cardiovasc Med. 2022 Jan 20;8:820416. doi: 10.3389/fcvm.2021.820416. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35127874 Free PMC article.
References
-
- Bassani R.A., Mattiazzi A., Bers D.M. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am. J. Physiol. 1995;268:H703–H712. - PubMed
-
- Karczewski P., Kuschel M., Baltas L.G., Bartel S., Krause E.G. Site-specific phosphorylation of a phospholamban peptide by cyclic nucleotide- and Ca2+/calmodulin-dependent protein kinases of cardiac sarcoplasmic reticulum. . Basic Res. Cardiol. 1997;92(Suppl. 1):37–43. - PubMed
-
- Witcher D.R., Kovacs R.J., Schulman H., Cefali D.C., Jones L.R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J. Biol. Chem. 1991;266:11144–11152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

