Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans
- PMID: 19381021
- PMCID: PMC2673881
- DOI: 10.1172/JCI38482
Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans
Abstract
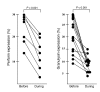
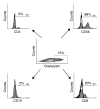
The incidence of tuberculosis is increased during treatment of autoimmune diseases with anti-TNF antibodies. This is a significant clinical complication, but also provides a unique model to study immune mechanisms in human tuberculosis. Given the key role for cell-mediated immunity in host defense against Mycobacterium tuberculosis, we hypothesized that anti-TNF treatment impairs T cell-directed antimicrobial activity. Anti-TNF therapy reduced the expression in lymphocytes of perforin and granulysin, 2 components of the T cell-mediated antimicrobial response to intracellular pathogens. Specifically, M. tuberculosis-reactive CD8+CCR7-CD45RA+ effector memory T cells (TEMRA cells) expressed the highest levels of granulysin, lysed M. tuberculosis, and infected macrophages and mediated an antimicrobial activity against intracellular M. tuberculosis. Furthermore, TEMRA cells expressed cell surface TNF and bound the anti-TNF therapeutic infliximab in vitro, making them susceptible to complement-mediated lysis. Immune therapy with anti-TNF was associated with reduced numbers of CD8+ TEMRA cells and decreased antimicrobial activity against M. tuberculosis, which could be rescued by the addition of CD8+ TEMRA cells. These results suggest that anti-TNF therapy triggers a reduction of CD8+ TEMRA cells with antimicrobial activity against M. tuberculosis, providing insight into the mechanism whereby key effector T cell subsets contribute to host defense against tuberculosis.
Figures

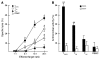
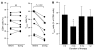

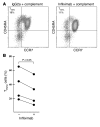

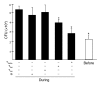
Comment in
-
Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed.J Clin Invest. 2009 May;119(5):1079-82. doi: 10.1172/jci39143. J Clin Invest. 2009. PMID: 19422095 Free PMC article.
References
-
- Maini R., et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

