Antiretroviral medication adherence and the development of class-specific antiretroviral resistance
- PMID: 19381075
- PMCID: PMC2704206
- DOI: 10.1097/QAD.0b013e32832ba8ec
Antiretroviral medication adherence and the development of class-specific antiretroviral resistance
Abstract
Objective: To assess the association between antiretroviral adherence and the development of class-specific antiretroviral medication resistance.
Design and methods: Literature and conference abstract review of studies assessing the association between adherence to antiretroviral therapy and the development of antiretroviral medication resistance.
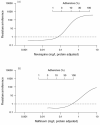
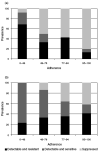
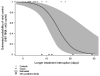
Results: Factors that determine class-specific adherence-resistance relationships include antiretroviral regimen potency, viral fitness or, more specifically, the interplay between the fold-change in resistance and fold-change in fitness caused by drug resistance mutations, and the genetic barrier to antiretroviral resistance. During multidrug therapy, differential drug exposure increases the likelihood of developing resistance. In addition, antiretroviral medications with higher potency and higher genetic barriers to resistance decrease the incidence of resistance for companion antiretroviral medications at all adherence levels.
Conclusion: Knowledge of class-specific adherence-resistance relationships may help clinicians and patients tailor therapy to match individual patterns of adherence in order to minimize the development of resistance at failure. In addition, this information may guide the selection of optimal drug combinations and regimen sequences to improve the durability of antiretroviral therapy.
Figures

References
-
- Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. - PubMed
-
- Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. - PubMed
-
- Preston BD, Poiesz BJ, Loeb LA. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. - PubMed
-
- Roberts JD, Bebenek K, Kunkel TA. The accuracy of reverse transcriptase from HIV-1. Science. 1988;242:1171–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

