Phoenix is required for mechanosensory hair cell regeneration in the zebrafish lateral line
- PMID: 19381250
- PMCID: PMC2662414
- DOI: 10.1371/journal.pgen.1000455
Phoenix is required for mechanosensory hair cell regeneration in the zebrafish lateral line
Abstract
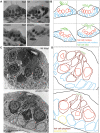
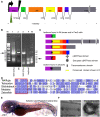
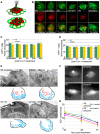
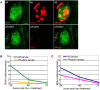
In humans, the absence or irreversible loss of hair cells, the sensory mechanoreceptors in the cochlea, accounts for a large majority of acquired and congenital hearing disorders. In the auditory and vestibular neuroepithelia of the inner ear, hair cells are accompanied by another cell type called supporting cells. This second cell population has been described as having stem cell-like properties, allowing efficient hair cell replacement during embryonic and larval/fetal development of all vertebrates. However, mammals lose their regenerative capacity in most inner ear neuroepithelia in postnatal life. Remarkably, reptiles, birds, amphibians, and fish are different in that they can regenerate hair cells throughout their lifespan. The lateral line in amphibians and in fish is an additional sensory organ, which is used to detect water movements and is comprised of neuroepithelial patches, called neuromasts. These are similar in ultra-structure to the inner ear's neuroepithelia and they share the expression of various molecular markers. We examined the regeneration process in hair cells of the lateral line of zebrafish larvae carrying a retroviral integration in a previously uncharacterized gene, phoenix (pho). Phoenix mutant larvae develop normally and display a morphologically intact lateral line. However, after ablation of hair cells with copper or neomycin, their regeneration in pho mutants is severely impaired. We show that proliferation in the supporting cells is strongly decreased after damage to hair cells and correlates with the reduction of newly formed hair cells in the regenerating phoenix mutant neuromasts. The retroviral integration linked to the phenotype is in a novel gene with no known homologs showing high expression in neuromast supporting cells. Whereas its role during early development of the lateral line remains to be addressed, in later larval stages phoenix defines a new class of proteins implicated in hair cell regeneration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nature Neurosci. Rev. 2006;7:837–849. - PubMed
-
- Kelley MW. Hair cell development: commitment through differentiation. Brain Res. 2006;1091:172–185. - PubMed
-
- Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nature Rev Neurosci. 2006;7:19–29. - PubMed
-
- Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

