Uric acid is a mediator of the Plasmodium falciparum-induced inflammatory response
- PMID: 19381275
- PMCID: PMC2667251
- DOI: 10.1371/journal.pone.0005194
Uric acid is a mediator of the Plasmodium falciparum-induced inflammatory response
Abstract
Background: Malaria triggers a high inflammatory response in the host that mediates most of the associated pathologies and contributes to death. The identification of pro-inflammatory molecules derived from Plasmodium is essential to understand the mechanisms of pathogenesis and to develop targeted interventions. Uric acid derived from hypoxanthine accumulated in infected erythrocytes has been recently proposed as a mediator of inflammation in rodent malaria.
Methods and findings: We found that human erythrocytes infected with Plasmodium falciparum gradually accumulate hypoxanthine in their late stages of development. To analyze the role of hypoxanthine-derived uric acid induced by P. falciparum on the inflammatory cytokine response from human blood mononuclear cells, cultures were treated with allopurinol, to inhibit uric acid formation from hypoxanthine, or with uricase, to degrade uric acid. Both treatments significantly reduce the secretion of TNF, IL-6, IL-1beta and IL-10 from human cells.
Conclusions and significance: Uric acid is a major contributor of the inflammatory response triggered by P. falciparum in human peripheral blood mononuclear cells. Since the inflammatory reaction induced by P. falciparum is considered a major cause of malaria pathogenesis, identifying the mechanisms used by the parasite to induce the host inflammatory response is essential to develop urgently needed therapies against this disease.
Conflict of interest statement
Figures
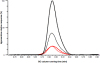

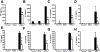
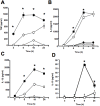
References
-
- Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. - PubMed
-
- Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

