Mechanisms involved in alleviation of intestinal inflammation by bifidobacterium breve soluble factors
- PMID: 19381276
- PMCID: PMC2667252
- DOI: 10.1371/journal.pone.0005184
Mechanisms involved in alleviation of intestinal inflammation by bifidobacterium breve soluble factors
Abstract
Objectives: Soluble factors released by Bifidobacterium breve C50 (Bb) alleviate the secretion of pro-inflammatory cytokines by immune cells, but their effect on intestinal epithelium remains elusive. To decipher the mechanisms accounting for the cross-talk between bacteria/soluble factors and intestinal epithelium, we measured the capacity of the bacteria, its conditioned medium (Bb-CM) and other Gram(+) commensal bacteria to dampen inflammatory chemokine secretion.
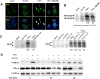
Methods: TNFalpha-induced chemokine (CXCL8) secretion and alteration of NF-kappaB and AP-1 signalling pathways by Bb were studied by EMSA, confocal microscopy and western blotting. Anti-inflammatory capacity was also tested in vivo in a model of TNBS-induced colitis in mice.
Results: Bb and Bb-CM, but not other commensal bacteria, induced a time and dose-dependent inhibition of CXCL8 secretion by epithelial cells driven by both AP-1 and NF-kappaB transcription pathways and implying decreased phosphorylation of p38-MAPK and IkappaB-alpha molecules. In TNBS-induced colitis in mice, Bb-CM decreased the colitis score and inflammatory cytokine expression, an effect reproduced by dendritic cell conditioning with Bb-CM.
Conclusions: Bb and secreted soluble factors contribute positively to intestinal homeostasis by attenuating chemokine production. The results indicate that Bb down regulate inflammation at the epithelial level by inhibiting phosphorylations involved in inflammatory processes and by protective conditioning of dendritic cells.
Conflict of interest statement
Figures




References
-
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–312. - PubMed
-
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. - PubMed
-
- McCole DF, Barrett KE. Varied role of the gut epithelium in mucosal homeostasis. Curr Opin Gastroenterol. 2007;23:647–654. - PubMed
-
- Lee J, Rachmilewitz D, Raz E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci. 2006;1072:351–355. - PubMed
-
- Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

