Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors
- PMID: 19381294
- PMCID: PMC2668171
- DOI: 10.1371/journal.pone.0005210
Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors
Abstract
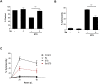
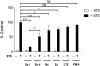
It is becoming increasingly apparent that Staphylococcus aureus are able to survive engulfment by macrophages, and that the intracellular environment of these host cells, which is essential to innate host defenses against invading microorganisms, may in fact provide a refuge for staphylococcal survival and dissemination. Based on this, we postulated that S. aureus might induce cytoprotective mechanisms by changing gene expression profiles inside macrophages similar to obligate intracellular pathogens, such as Mycobacterium tuberculosis. To validate our hypothesis we first ascertained whether S. aureus infection could affect programmed cell death in human (hMDMs) and mouse (RAW 264.7) macrophages and, specifically, protect these cells against apoptosis. Our findings indicate that S. aureus-infected macrophages are more resistant to staurosporine-induced cell death than control cells, an effect partly mediated via the inhibition of cytochrome c release from mitochondria. Furthermore, transcriptome analysis of human monocyte-derived macrophages during S. aureus infection revealed a significant increase in the expression of antiapoptotic genes. This was confirmed by quantitative RT-PCR analysis of selected genes involved in mitochondria-dependent cell death, clearly showing overexpression of BCL2 and MCL1. Cumulatively, the results of our experiments argue that S. aureus is able to induce a cytoprotective effect in macrophages derived from different mammal species, which can prevent host cell elimination, and thus allow intracellular bacterial survival. Ultimately, it is our contention that this process may contribute to the systemic dissemination of S. aureus infection.
Conflict of interest statement
Figures


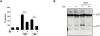
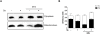

References
-
- Archer GL. Staphylococus aureus: A well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. - PubMed
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. - PubMed
-
- Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. - PubMed
-
- Liñares J. The VISA/GISA problem: therapeutic implication. Clin Microbiol Infect. 2001;7:8–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

