Uncovering intrinsic modular organization of spontaneous brain activity in humans
- PMID: 19381298
- PMCID: PMC2668183
- DOI: 10.1371/journal.pone.0005226
Uncovering intrinsic modular organization of spontaneous brain activity in humans
Abstract
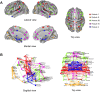
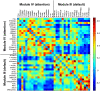
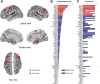
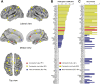
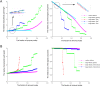
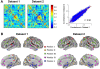
The characterization of topological architecture of complex brain networks is one of the most challenging issues in neuroscience. Slow (<0.1 Hz), spontaneous fluctuations of the blood oxygen level dependent (BOLD) signal in functional magnetic resonance imaging are thought to be potentially important for the reflection of spontaneous neuronal activity. Many studies have shown that these fluctuations are highly coherent within anatomically or functionally linked areas of the brain. However, the underlying topological mechanisms responsible for these coherent intrinsic or spontaneous fluctuations are still poorly understood. Here, we apply modern network analysis techniques to investigate how spontaneous neuronal activities in the human brain derived from the resting-state BOLD signals are topologically organized at both the temporal and spatial scales. We first show that the spontaneous brain functional networks have an intrinsically cohesive modular structure in which the connections between regions are much denser within modules than between them. These identified modules are found to be closely associated with several well known functionally interconnected subsystems such as the somatosensory/motor, auditory, attention, visual, subcortical, and the "default" system. Specifically, we demonstrate that the module-specific topological features can not be captured by means of computing the corresponding global network parameters, suggesting a unique organization within each module. Finally, we identify several pivotal network connectors and paths (predominantly associated with the association and limbic/paralimbic cortex regions) that are vital for the global coordination of information flow over the whole network, and we find that their lesions (deletions) critically affect the stability and robustness of the brain functional system. Together, our results demonstrate the highly organized modular architecture and associated topological properties in the temporal and spatial brain functional networks of the human brain that underlie spontaneous neuronal dynamics, which provides important implications for our understanding of how intrinsically coherent spontaneous brain activity has evolved into an optimal neuronal architecture to support global computation and information integration in the absence of specific stimuli or behaviors.
Conflict of interest statement
Figures



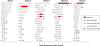
References
-
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. - PubMed
-
- Raichle ME. Neuroscience. The brain's dark energy. Science. 2006;314:1249–1250. - PubMed
-
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. - PubMed
-
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. - PubMed
-
- He Y, Wang L, Zang Y, Tian L, Zhang X, et al. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

