Potential Hepatotoxicity of Efavirenz and Saquinavir/Ritonavir Coadministration in Healthy Volunteers
- PMID: 19381337
- PMCID: PMC2667896
- DOI: 10.1111/j.1753-5174.2009.00016.x
Potential Hepatotoxicity of Efavirenz and Saquinavir/Ritonavir Coadministration in Healthy Volunteers
Abstract
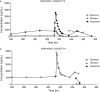
OBJECTIVE: This study was designed to investigate the pharmacokinetic effects of coadministration of saquinavir/ritonavir with efavirenz at steady state. METHODS: Healthy volunteers in this open-label, two-arm, one-sequence, two-period crossover study (planned enrollment of 40 participants) were randomized to one of two treatment arms: those in Arm 1 were scheduled to receive saquinavir/ritonavir 1,000/100 mg orally twice daily for 29 days and efavirenz 600 mg orally once daily starting on day 15 and continuing through day 29; participants randomized to Arm 2 were to receive efavirenz once daily for 29 days and saquinavir/ritonavir 1,000/100 mg twice daily starting on day 15 through day 29. Assessments included vital signs, laboratory analyses, electrocardiography, and blood levels of total saquinavir, ritonavir, and efavirenz. Pharmacokinetic parameters included C(max) (maximum observed plasma concentration), t(max) (time to reach the maximum observed plasma concentration), (apparent elimination half-life), and AUC(0-tau) (area-under-the-plasma-concentration-time curve over one dosing interval). RESULTS: Eight participants (four in each arm) were enrolled; only two (one from each treatment arm) reached day 15 of the study and received the concurrent initial doses of saquinavir/ritonavir and efavirenz. The study was terminated prematurely after these two participants experienced nonserious adverse events. The participant in Arm 1 experienced mild abdominal discomfort, diarrhea, sleep disorder, and headache and the participant in Arm 2 experienced moderate-intensity abdominal pain and mild vomiting with leukocytosis accompanied by elevated pancreatic and hepatic enzymes (aspartate aminotransferase and alanine aminotransferase values of 2-fold and 3.5-fold the upper limit of normal, respectively). Both participants recovered completely following treatment discontinuation. Only limited pharmacokinetic data were generated on these two participants. CONCLUSIONS: The early termination of this study precluded drawing any definitive conclusions regarding the pharmacokinetics at steady state of coadministered saquinavir/ritonavir and efavirenz.
Figures
Similar articles
-
Unexpected Hepatotoxicity of Rifampin and Saquinavir/Ritonavir in Healthy Male Volunteers.Arch Drug Inf. 2009 Mar;2(1):8-16. doi: 10.1111/j.1753-5174.2009.00017.x. Arch Drug Inf. 2009. PMID: 19381336 Free PMC article.
-
Once-daily dosing of saquinavir and low-dose ritonavir in HIV-1-infected individuals: a pharmacokinetic pilot study.AIDS. 2000 Jun 16;14(9):F103-10. doi: 10.1097/00002030-200006160-00003. AIDS. 2000. PMID: 10894270 Clinical Trial.
-
Effect of saquinavir-ritonavir on cytochrome P450 3A4 activity in healthy volunteers using midazolam as a probe.Pharmacotherapy. 2009 Oct;29(10):1175-81. doi: 10.1592/phco.29.10.1175. Pharmacotherapy. 2009. PMID: 19792991
-
Amprenavir or fosamprenavir plus ritonavir in HIV infection: pharmacology, efficacy and tolerability profile.Drugs. 2005;65(5):633-59. doi: 10.2165/00003495-200565050-00005. Drugs. 2005. PMID: 15748098 Review.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
Cited by
-
Evaluation of Drug-Drug Interactions between Direct-Acting Anti-Hepatitis C Virus Combination Regimens and the HIV-1 Antiretroviral Agents Raltegravir, Tenofovir, Emtricitabine, Efavirenz, and Rilpivirine.Antimicrob Agents Chemother. 2016 Apr 22;60(5):2965-71. doi: 10.1128/AAC.02605-15. Print 2016 May. Antimicrob Agents Chemother. 2016. PMID: 26953200 Free PMC article.
-
Targeting Xenobiotic Nuclear Receptors PXR and CAR to Prevent Cobicistat Hepatotoxicity.Toxicol Sci. 2021 Apr 27;181(1):58-67. doi: 10.1093/toxsci/kfab023. Toxicol Sci. 2021. PMID: 33629115 Free PMC article.
-
PXR-mediated idiosyncratic drug-induced liver injury: mechanistic insights and targeting approaches.Expert Opin Drug Metab Toxicol. 2020 Aug;16(8):711-722. doi: 10.1080/17425255.2020.1779701. Epub 2020 Jun 16. Expert Opin Drug Metab Toxicol. 2020. PMID: 32500752 Free PMC article. Review.
-
Metabolomic screening and identification of the bioactivation pathways of ritonavir.Chem Res Toxicol. 2011 Dec 19;24(12):2109-14. doi: 10.1021/tx2004147. Epub 2011 Nov 17. Chem Res Toxicol. 2011. PMID: 22040299 Free PMC article.
-
Pregnane X receptor activation potentiates ritonavir hepatotoxicity.J Clin Invest. 2019 Apr 30;129(7):2898-2903. doi: 10.1172/JCI128274. J Clin Invest. 2019. PMID: 31039134 Free PMC article.
References
-
- Sustiva® (Efavirenz) Capsules and Tablets. [accessed October 3, 2008]. Available at: http://packageinserts.bms.com/pi/pi_sustiva.pdf.
-
- Falloon J, Piscitelli S, Vogel S, et al. Combination therapy with amprenavir, abacavir, and efavirenz in human immunodeficiency virus (HIV)-infected patients failing a protease-inhibitor regimen: Pharmacokinetic drug interactions and antiviral activity. Clin Infect Dis. 2000;30:313–8. - PubMed
-
- Malaty LI, Kuper JJ. Drug interactions of HIV protease inhibitors. Drug Saf. 1999;20:147–69. - PubMed
-
- Merry C, Barry MG, Mulcahy F, et al. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS. 1997;11:F29–33. - PubMed
-
- Koudriakova T, Iatsimirskaia E, Utkin I, et al. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: Mechanism-based inactivation of cytochrome P4503A by ritonavir. Drug Metab Dispos. 1998;26:552–61. - PubMed
LinkOut - more resources
Full Text Sources
