Role of spike-frequency adaptation in shaping neuronal response to dynamic stimuli
- PMID: 19381681
- PMCID: PMC2854487
- DOI: 10.1007/s00422-009-0304-y
Role of spike-frequency adaptation in shaping neuronal response to dynamic stimuli
Abstract
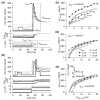
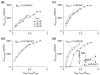
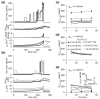
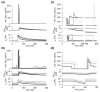
Spike-frequency adaptation is the reduction of a neuron's firing rate to a stimulus of constant intensity. In the locust, the Lobula Giant Movement Detector (LGMD) is a visual interneuron that exhibits rapid adaptation to both current injection and visual stimuli. Here, a reduced compartmental model of the LGMD is employed to explore adaptation's role in selectivity for stimuli whose intensity changes with time. We show that supralinearly increasing current injection stimuli are best at driving a high spike count in the response, while linearly increasing current injection stimuli (i.e., ramps) are best at attaining large firing rate changes in an adapting neuron. This result is extended with in vivo experiments showing that the LGMD's response to translating stimuli having a supralinear velocity profile is larger than the response to constant or linearly increasing velocity translation. Furthermore, we show that the LGMD's preference for approaching versus receding stimuli can partly be accounted for by adaptation. Finally, we show that the LGMD's adaptation mechanism appears well tuned to minimize sensitivity for the level of basal input.
Figures



Similar articles
-
Spike frequency adaptation mediates looming stimulus selectivity in a collision-detecting neuron.Nat Neurosci. 2009 Mar;12(3):318-26. doi: 10.1038/nn.2259. Epub 2009 Feb 8. Nat Neurosci. 2009. PMID: 19198607 Free PMC article.
-
Fine and distributed subcellular retinotopy of excitatory inputs to the dendritic tree of a collision-detecting neuron.J Neurophysiol. 2016 Jun 1;115(6):3101-12. doi: 10.1152/jn.00044.2016. Epub 2016 Mar 23. J Neurophysiol. 2016. PMID: 27009157 Free PMC article.
-
Spike-frequency adaptation and intrinsic properties of an identified, looming-sensitive neuron.J Neurophysiol. 2006 Dec;96(6):2951-62. doi: 10.1152/jn.00075.2006. Epub 2006 Mar 29. J Neurophysiol. 2006. PMID: 16571737 Free PMC article.
-
Synchronized neural input shapes stimulus selectivity in a collision-detecting neuron.Curr Biol. 2010 Nov 23;20(22):2052-7. doi: 10.1016/j.cub.2010.10.025. Epub 2010 Nov 4. Curr Biol. 2010. PMID: 21055939 Free PMC article.
-
Shaping the collision selectivity in a looming sensitive neuron model with parallel ON and OFF pathways and spike frequency adaptation.Neural Netw. 2018 Oct;106:127-143. doi: 10.1016/j.neunet.2018.04.001. Epub 2018 Apr 16. Neural Netw. 2018. PMID: 30059829
Cited by
-
A Looming Spatial Localization Neural Network Inspired by MLG1 Neurons in the Crab Neohelice.Front Neurosci. 2022 Jan 21;15:787256. doi: 10.3389/fnins.2021.787256. eCollection 2021. Front Neurosci. 2022. PMID: 35126038 Free PMC article.
-
Sex Differences in Biophysical Signatures across Molecularly Defined Medial Amygdala Neuronal Subpopulations.eNeuro. 2020 Jul 2;7(4):ENEURO.0035-20.2020. doi: 10.1523/ENEURO.0035-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32493755 Free PMC article.
-
Adiabatic dynamic causal modelling.Neuroimage. 2021 Sep;238:118243. doi: 10.1016/j.neuroimage.2021.118243. Epub 2021 Jun 8. Neuroimage. 2021. PMID: 34116151 Free PMC article.
-
Selective attenuation of Ether-a-go-go related K+ currents by endogenous acetylcholine reduces spike-frequency adaptation and network correlation.Elife. 2019 Apr 29;8:e44954. doi: 10.7554/eLife.44954. Elife. 2019. PMID: 31032798 Free PMC article.
-
How noisy adaptation of neurons shapes interspike interval histograms and correlations.PLoS Comput Biol. 2010 Dec 16;6(12):e1001026. doi: 10.1371/journal.pcbi.1001026. PLoS Comput Biol. 2010. PMID: 21187900 Free PMC article.
References
-
- Benda J, Hennig RM. Spike-frequency adaptation generates intensity invariance in a primary auditory interneuron. J Comput Neurosci. 2008;24:113–136. - PubMed
-
- Benda J, Herz AV. A universal model for spike-frequency adaptation. Neural Comput. 2003;15:2523–2564. - PubMed
-
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. - PubMed
-
- Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

