Formulation and performance characterization of radio-sterilized "progestin-only" microparticles intended for contraception
- PMID: 19381829
- PMCID: PMC2690789
- DOI: 10.1208/s12249-009-9226-1
Formulation and performance characterization of radio-sterilized "progestin-only" microparticles intended for contraception
Abstract
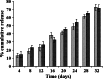
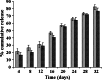
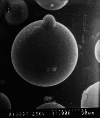
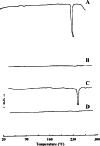
The aim of this study was to formulate and characterize a microparticulate system of progestin-only contraceptive. Another objective was to evaluate the effect of gamma radio-sterilization on in vitro and in vivo drug release characteristics. Levonorgestrel (LNG) microspheres were fabricated using poly(lactide-co-glycolide) (PLGA) by a novel solvent evaporation technique. The formulation was optimized for drug/polymer ratio, emulsifier concentration, and process variables like speed of agitation and evaporation method. The drug to polymer ratio of 1:5 gave the optimum encapsulation efficiency. Speed of agitation influenced the spherical shape of the microparticles, lower speeds yielding less spherical particles. The speed did not have a significant influence on the drug payloads. A combination of stabilizers viz. methyl cellulose and poly vinyl alcohol with in-water solvent evaporation technique yielded microparticles without any free drug crystals on the surface. This aspect significantly eliminated the in vitro dissolution "burst effect". The residual solvent content was well within the regulatory limits. The microparticles passed the test for sterility and absence of pyrogens. In vitro dissolution conducted on the product before and after gamma radiation sterilization at 2.5 Mrad indicated no significant difference in the drug release patterns. The drug release followed zero-order kinetics in both static and agitation conditions of dissolution testing. The in vivo studies conducted in rabbits exhibited LNG release up to 1 month duration with drug levels maintained within the effective therapeutic window.
Figures
Similar articles
-
Gamma irradiated micro system for long-term parenteral contraception: An alternative to synthetic polymers.Eur J Pharm Sci. 2008 Nov 15;35(4):307-17. doi: 10.1016/j.ejps.2008.07.009. Epub 2008 Aug 8. Eur J Pharm Sci. 2008. PMID: 18760352
-
Fabrication, characterization and in vivo studies of biodegradable gamma sterilized injectable microparticles for contraception.Pharm Dev Technol. 2009;14(3):278-89. doi: 10.1080/10837450802585260. Pharm Dev Technol. 2009. PMID: 19235552
-
Controlled release of levonorgestrel from biodegradable poly(D,L-lactide-co-glycolide) microspheres: in vitro and in vivo studies.Int J Pharm. 2005 Sep 14;301(1-2):217-25. doi: 10.1016/j.ijpharm.2005.05.038. Int J Pharm. 2005. PMID: 16040213
-
Biodegradable PLGA microspheres loaded with ganciclovir for intraocular administration. Encapsulation technique, in vitro release profiles, and sterilization process.Pharm Res. 2000 Oct;17(10):1323-8. doi: 10.1023/a:1026464124412. Pharm Res. 2000. PMID: 11145241
-
Recent Progress in Drug Release Testing Methods of Biopolymeric Particulate System.Pharmaceutics. 2021 Aug 23;13(8):1313. doi: 10.3390/pharmaceutics13081313. Pharmaceutics. 2021. PMID: 34452274 Free PMC article. Review.
Cited by
-
Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems.Pharmaceutics. 2019 Mar 21;11(3):140. doi: 10.3390/pharmaceutics11030140. Pharmaceutics. 2019. PMID: 30901930 Free PMC article. Review.
-
Local administration of irinotecan using an implantable drug delivery device stops high-grade glioma tumor recurrence in a glioblastoma tumor model.Drug Deliv Transl Res. 2024 Nov;14(11):3070-3088. doi: 10.1007/s13346-024-01524-x. Epub 2024 Feb 6. Drug Deliv Transl Res. 2024. PMID: 38319555 Free PMC article.
-
Qualification of Non-Halogenated Organic Solvents Applied to Microsphere Manufacturing Process.Pharmaceutics. 2020 May 6;12(5):425. doi: 10.3390/pharmaceutics12050425. Pharmaceutics. 2020. PMID: 32384751 Free PMC article.
-
Dexamethasone PLGA Microspheres for Sub-Tenon Administration: Influence of Sterilization and Tolerance Studies.Pharmaceutics. 2021 Feb 6;13(2):228. doi: 10.3390/pharmaceutics13020228. Pharmaceutics. 2021. PMID: 33562155 Free PMC article.
References
-
- Heya T, Okada H, Ogawa Y, Toguchi H. Factors influencing the profiles of TRH release from copoly(d,l-lactic/glycolic acid) microspheres. Int J Pharm. 1991;72:199–205. doi: 10.1016/0378-5173(91)90108-Z. - DOI
-
- Sah HK, Chien YW. Evaluation of a microreservoir-type biodegradable microcapsule for controlled release of proteins. Drug Dev Ind Pharm. 1993;19:1243–63. doi: 10.3109/03639049309074399. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

